ATP 3
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
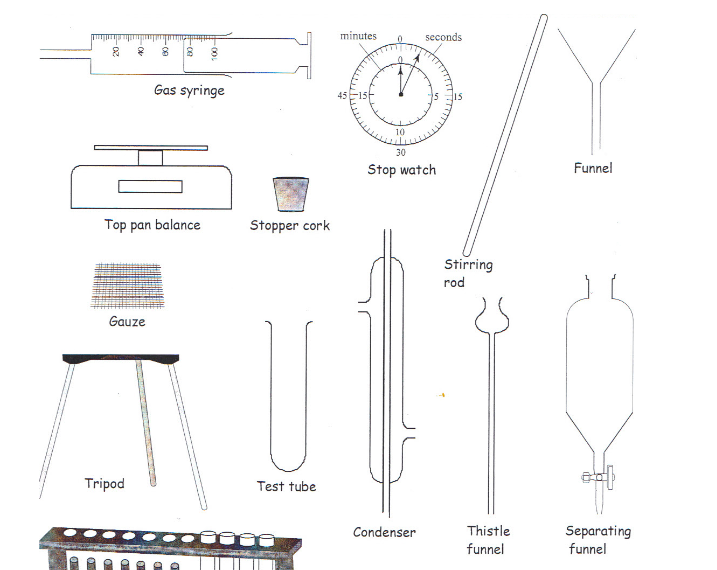
apparatus one
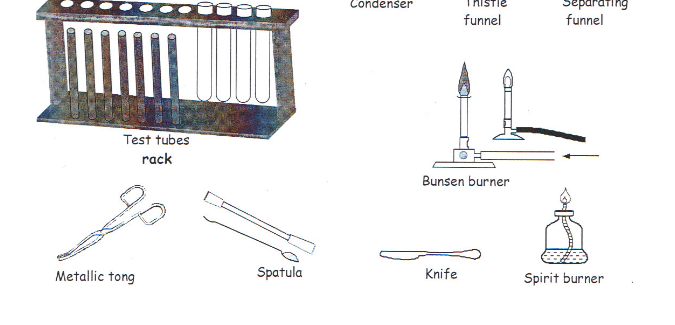
apparatus two
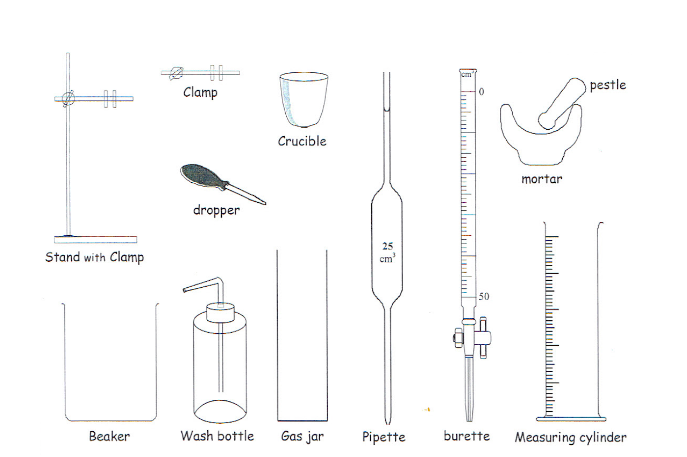
apparatus three
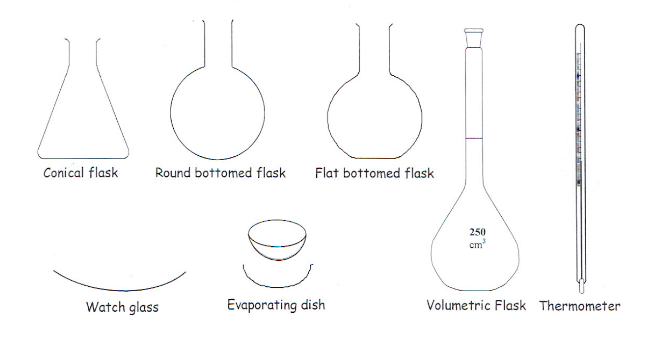
apparatus four
upward delivery
collect gas lighter than air
ammonia and hydrogen
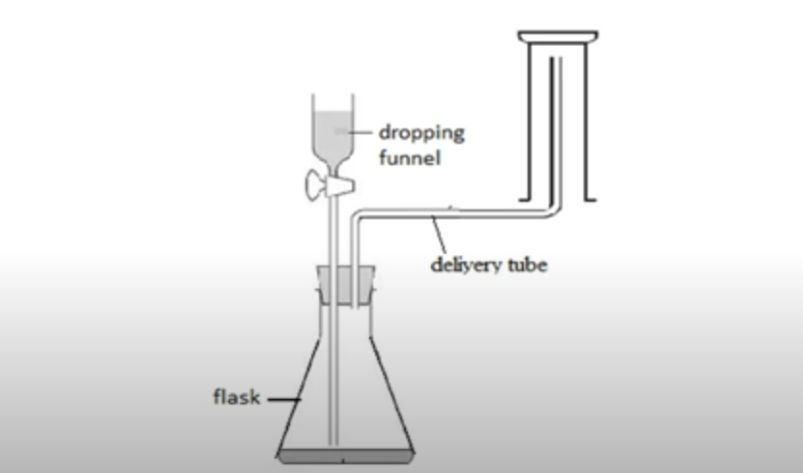
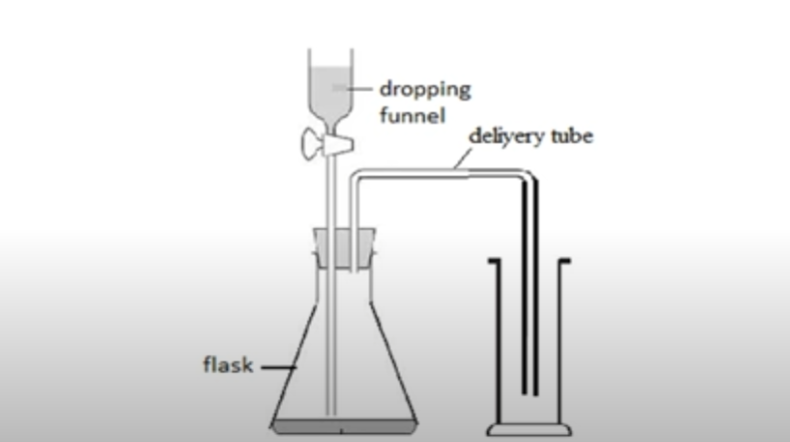
downward delivery
collect gases heavier than air
carbon dioxide, oxygen, etc
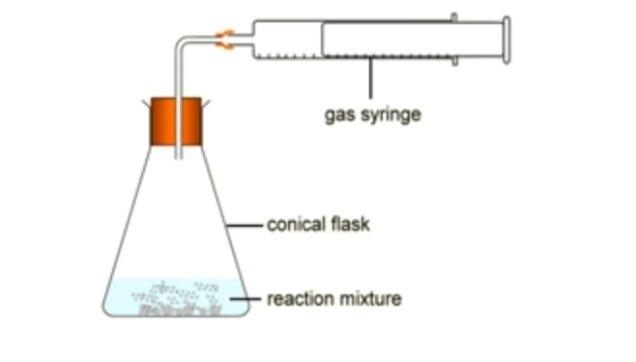
determine volume of gas
use gas syringe
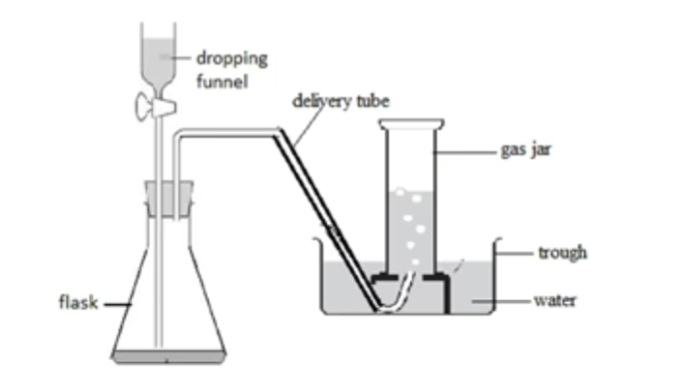
Over water
Carbon dioxide, hydrogen, oxygen Done when: Gas is insoluble in water |
cracking precautions
Delivery tube must be removed before heating is stopped to prevent back suction of the water into the hot tube which would break it.
Universal indicator
More accurate gas jar?
Gas syringe
Dry a solid
Dry it between filter papers
Why not heat crystals in an oven?
this may cause decomposition of the substance or loss of water of crystallization.
Burrete or pippete?
Burrete for greater than 25cm³
max volume of burette is 50cm³
volume and temperature graphs for endothermic and exothermic reactions
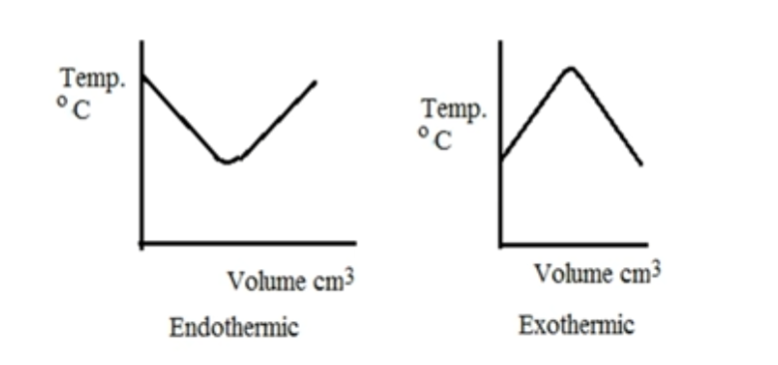
improvement if polystyrene is already used
use a lid or insulate the apparatus
how to know all liquid has been collected in distillation
temperature starts to rise
fuel experiment errors
Loss of heat to the surroundings.
Incomplete combustion of the fuel.
Insulate the experiment to prevent loss of heat to the environment.
Use copper can instead of a test tube.
Why can’t we put our baseline in solvent?
dyes would mix / dissolve with solvent / wash off paper
calculate volume of CO2 from fizzy drinks
Measure 25 cm³ of the drink using a pipette. Put into a flask connected to a gas syringe. Heat the flask until no more încrease "in volume of gas in the gas syringe: Determine the highest volume of gas collected. Multiply by 40 (to get 1000cm³) to determine the volume that would be collected from 1000 cm³