rp4 - cations and anions
1/23
Earn XP
Description and Tags
results of halide ions w/ conc sulfuric acid in halide ions fcs
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
describe how we can use dilute NaOH to identify group 2 cations:
add 6 drops of dilute NaOH solution to test tube containing metal ion solution (e.g. magnesium sulfate, Mg2+ etc.) and observe precipitate (if any) formed
keep adding dilute NaOH until it is in excess - record any changes you see
give 3 ways we can identify group 2 metal cations:
flame test (not in RP4)
by adding dilute NaOH
by adding H2SO4
what is seen for each of the group 2 metal cations when dilute NaOH is added?
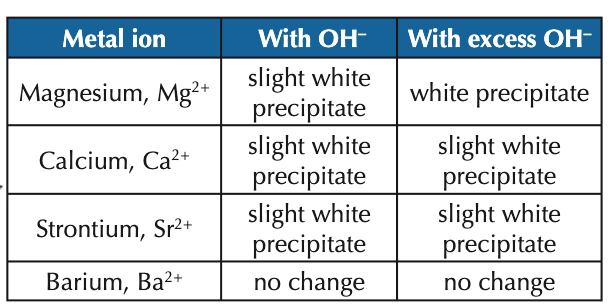
what would be the initial observation for a group 2 metal compound?
colourless solution
how can we test for ammonium (NH4+) ions?
add substance you are testing to test tube
add NaOH solution and shake
warm gently using a water bath
ammonia should be released - test fumes released by holding damp litmus paper at mouth of test tube
if ammonium ions present, damp litmus paper turns from red → blue
give the symbol eqn for the ammonium ion test:
NH4+ (aq) + OH- (aq) → NH3 (g) + H2O (l)
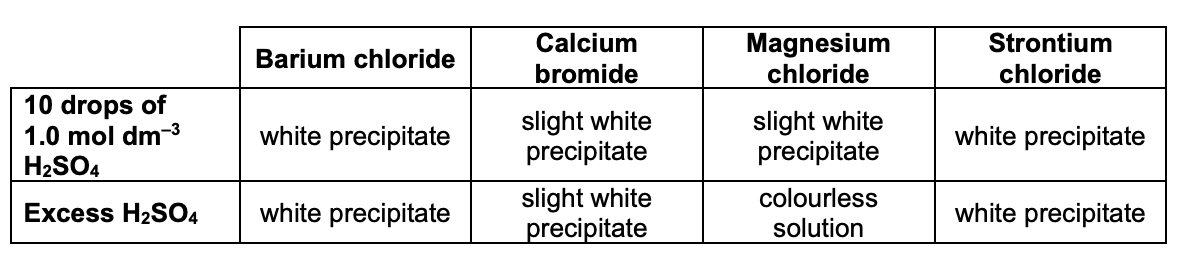
describe how we can use H2SO4 to identify group 2 metal cations:
add 10 drops of H2SO4 solution to test tube containing metal ion solution (e.g. magnesium sulfate, Mg2+ etc.) and observe precipitate (if any) formed
keep adding H2SO4 until it is in excess - record any changes you see
what is seen for each of the group 2 metal ions when H2SO4 is added?
give 2 ways we can test for OH- ions:
using litmus paper
using ammonia solution and litmus paper
how can we test for OH- ions with just red litmus paper?
dip a piece of red litmus paper into the solution
if OH- ions are present, the paper will turn blue
how can we test for OH- ions using ammonia solution and litmus paper?
take 5 drops of ammonia solution and place on filter paper
place this inside a petri dish w/ a lid
dampen a piece of red litmus paper w/ deionised water and place on other side of petri dish
replace lid and observe over a few minutes - if OH- ions present, litmus paper should turn blue as ammonia vapours produced
how does the test for OH- ions and litmus paper work?
we add deionised water as OH- ions form when NH3 comes into contact w/ water
this turns red litmus paper blue as ammonia solution vapours are alkaline
how can we test for CO32- ions in aqueous solution?
add 2 cm³ Ca(OH)2 (limewater) to a test tube
to a different test tube, add 3 cm³ Na2CO3 and an equal volume of dilute HCl
immediately place a delivery tube from this to the limewater test tube
if CO32- ions present, limewater goes cloudy
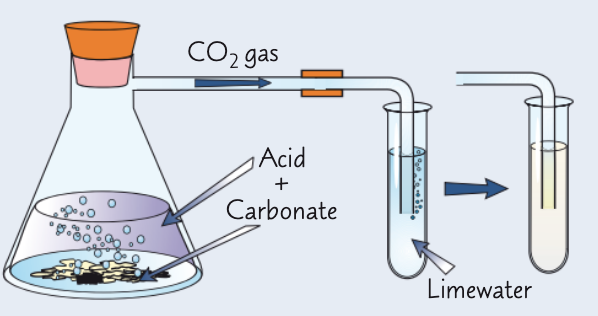
explain how HCl can help detect CO32- ions and give the eqn:
limewater turns cloudy if carbonate ions present as CO2 is produced in the reaction w/ dilute HCl (H+ ions)
CO32-(aq) + 2H+ (aq) → CO2 (g) + H2O (l)
how can we test for sulfate ions in aqueous solution?
add dilute HCl and acidified BaCl2
white precipitate of BaSO4 forms if SO42- ions present
(BaCl2 acidified by HCl but is in MS)
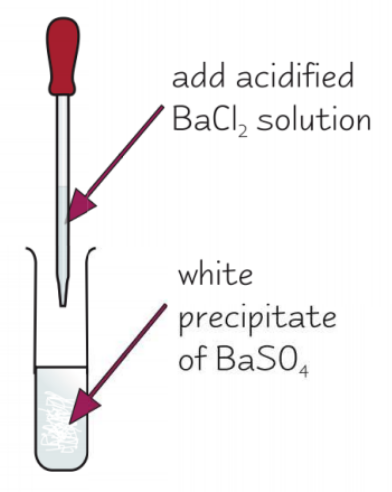
give the eqn for the test for sulfate ions in aqueous solution:
Ba2+ (aq) + SO42- (aq) → BaSO4 (s)
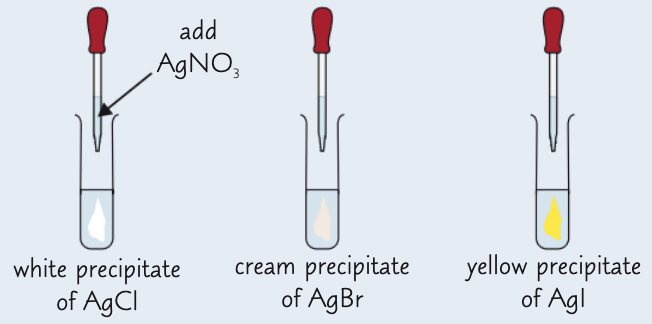
how can we test for halide ions in aqueous solution?
add 10 drops of substance you are testing to a clean, dry test tube
add approx 5 drops of dilute nitric acid and shake
add 10 drops silver nitrate solution and record observations
add dilute ammonia (or ammonia solution if testing for I- ions and if so, work in a fume cupboard) and record further observations
why do we add nitric acid during the test for halide ions?
to remove CO32- and OH- ions, which would also form precipitates and interfere w/ the test
give the general eqn for the reaction of halide ions w/ silver nitrate:
Ag+ (aq) + X- (aq) → AgX (s)
give the results of the silver nitrate test:
AgCl - white ppt
AgBr - cream ppt
AgI - yellow ppt
give the results of the silver nitrate halide ions test when aqueous ammonia/ammonia solution is added:
AgCl - redissolves
AgBr - redissolves slowly and needs a lot of ammonia
AgI - does not dissolve
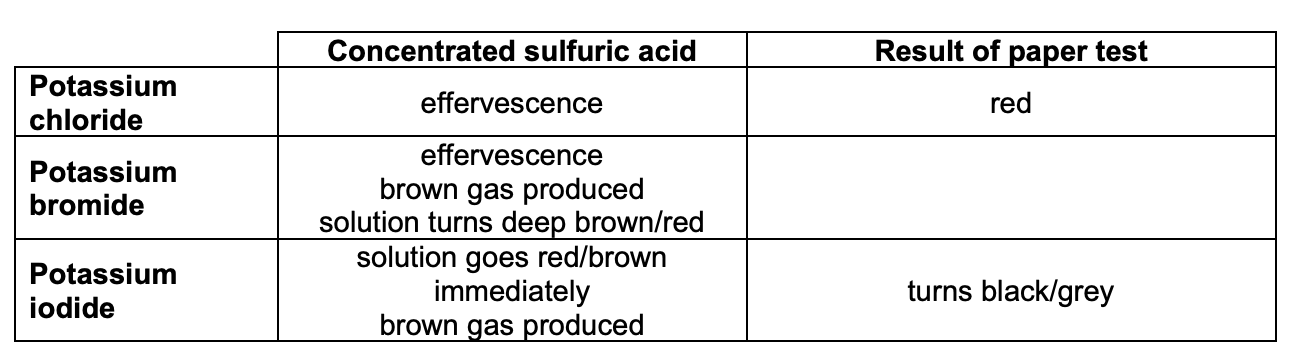
how can we test for halide ions in solid salts?
carry out in a fume cupboard and wear gloves:
place a small spatula of the solid you are testing into a clean, dry test tube
slowly add a few drops of concentrated H2SO4 and record observations
test gas w/ moist blue litmus paper and record observations
(repeat w/ acidified K2Cr2O7 / lead (II) nitrate solution)
give the observations for the tests for halide ions in solid salts:
(not part of the practical but useful!) how can we test for all acids? what denotes a +ve result?
add Na2CO3
+ve result = effervescence - as CO2 produced