Periodic Table
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Isotype definition
Atoms with the same amount of protons but different numbers of neutrons
Relative charge and mass of a proton
RC= +1 RM= 1
Relative charge and mass of a neutron
RC= 0 RM= 1
Relative charge and mass of electron
RC=-1 RM=0
How to find the number of protons?
Use atomic number- bottom of symbol
What did Dalton discover?
solid sphere- atom
What did Thompson discover?
plum pudding- electron
What did Rutherford discover?
nucleus- positive nucleus surrounded electrons
What did Chadwick discover?
neutron- planetary model- electrons orbit
What was the periodic table first ordered by?
Mendeleev- atomic mass and chemical properties- predicted not discovered elements
Properties of metals
High density, strong, malleable, good heat and electricity conduction
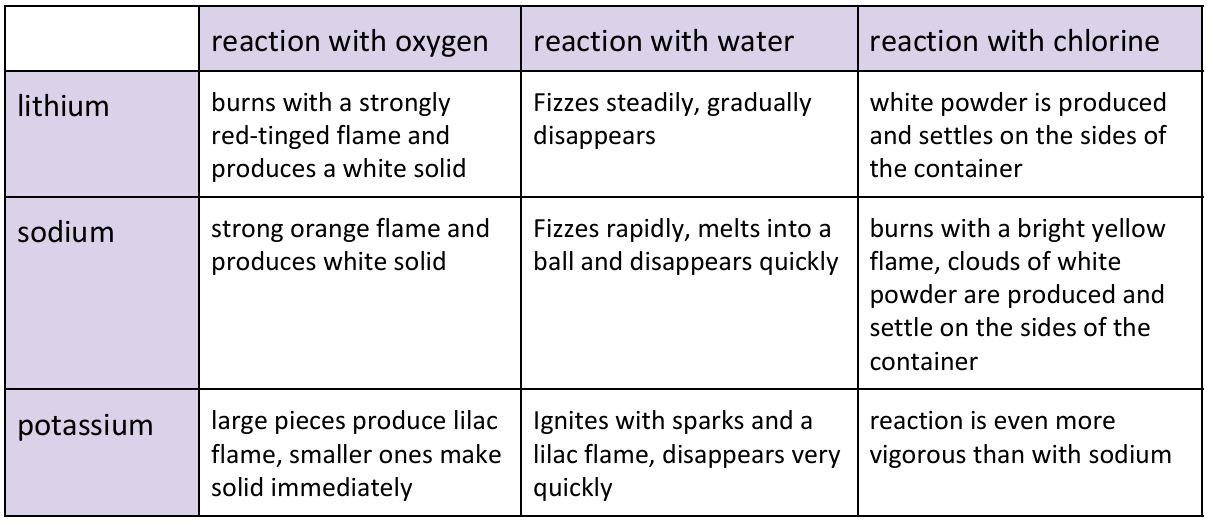
group 1, react with water to create alkaline solution and hydrogen, react with oxygen to create oxide, reacts with chlorine to form white precipitate
Properties of non-metals
Low density, weak, brittle, poor conductors of heat and electricity
group 7 (halogens)
react with metal to form halide ions -1 charge
reactivity decreases down- more reactive halogen can displace less reactive in an aqueous solution of its salt
chlorine will displace bromine in potassium bromine
Trends as you go down group 1
Melting point decreases
Density increases
Reactivity increases
Trends as you go down group 7
Melting point increases
Density increases
Reactivity decreases
Trends as you go down the noble gases
Melting point increases
Density increases
NO REACTIVITY
How is chromatography used as a separation technique?
Used to separate a mixture of dyes in ink
How is filtration used as a separation technique?
Used to separate insoluble solids from liquids
How is evaporation used as a separation technique?
Used to separate a soluble salt from solution. Solution heated strongly in evaporating basin till dry crystals are left
How is crystallisation used as a separation technique?
Separate soluble salt from solution. Solution heated gently in evaporating basin till crystals form
How is simple distillation used as a separation technique?
Separate liquid from solution- condenser used to cool hot gas till forms a liquid
How is fractional distillation used as a separation technique?
Used to separate a mixture of liquids with different boiling points