LIPIDS AND LIPOPROTEINS
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
What are the major functions of lipids and what diseases result from lipid imbalance?
Major source of energy (9 kcal/g)
Used in energy production as fatty acids
Hydrophobic barrier for subcellular compartmentalization
Regulators/cofactors (fat‑soluble vitamins)
Precursors of prostaglandins and steroid hormones
Stored in adipose tissue as triglycerides
Must be transported in lipoproteins
Disorders from imbalance: Obesity, Atherosclerosis → contribute to metabolic syndrome
What types of lipids are included in dietary lipid intake?
Triglycerides (esterified fatty acids)
Unesterified fatty acids (free fatty acids)
Cholesterol
Cholesteryl esters
Phospholipids
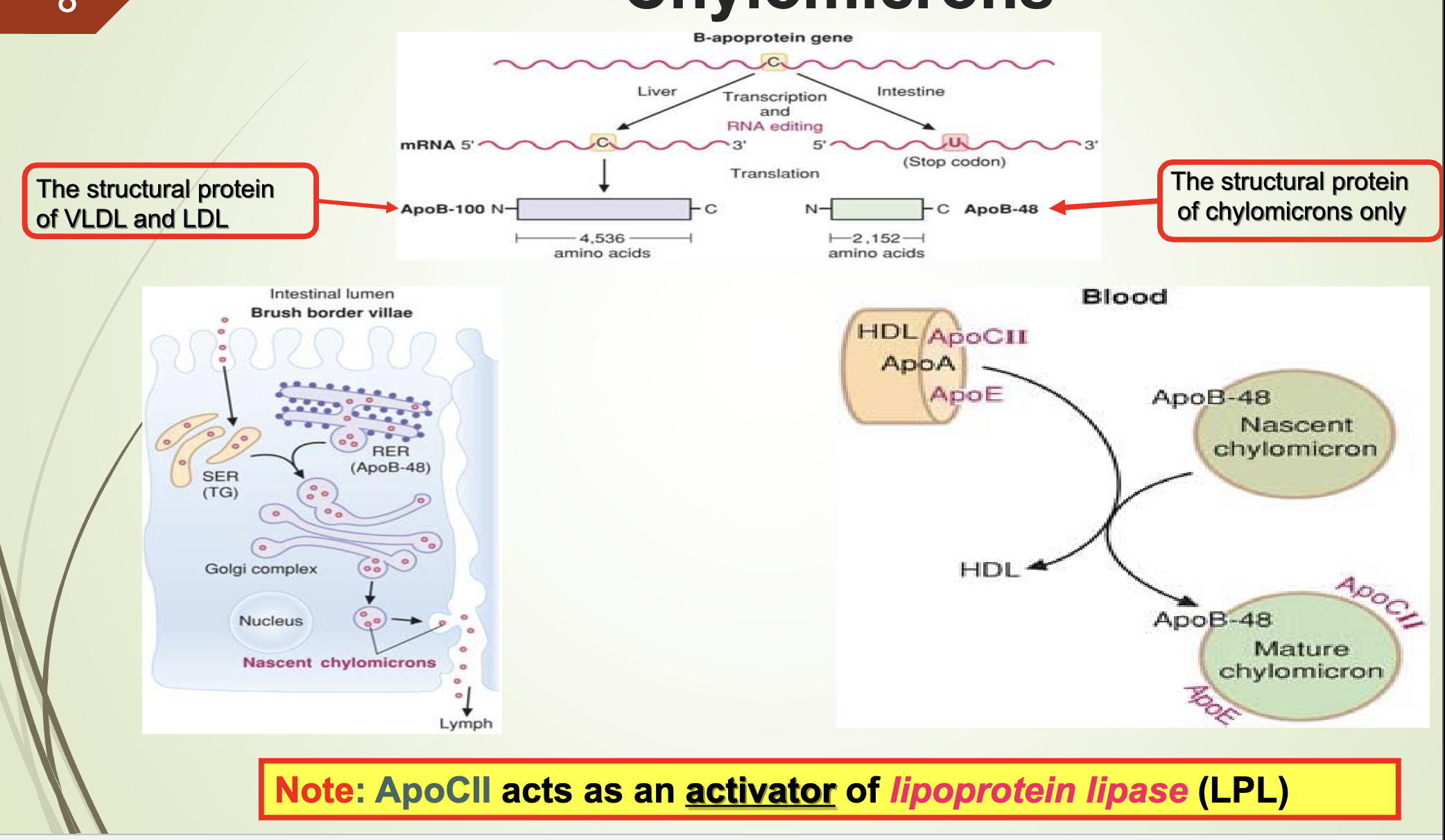
Where are chylomicrons synthesized and from what components?
Synthesized in intestinal mucosal cells
Made from dietary lipids and apoprotein B‑48
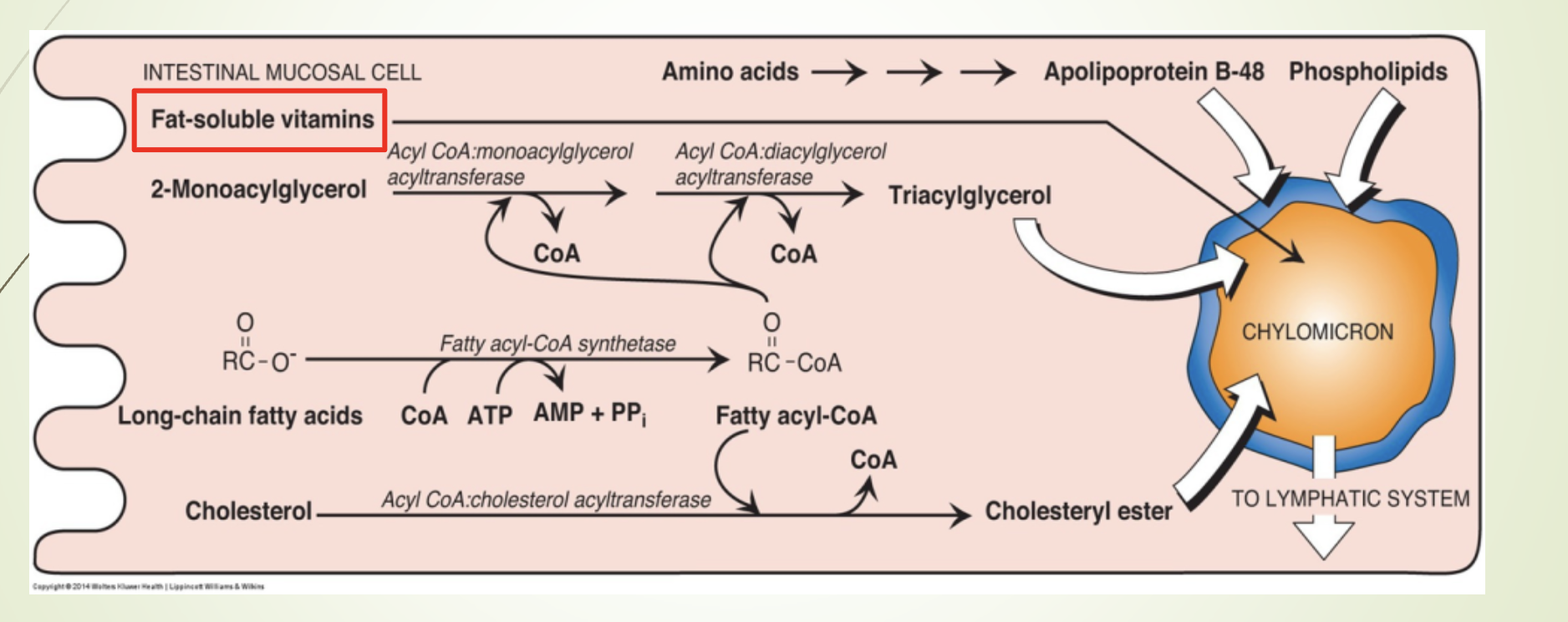
What is the function and composition of chylomicrons?
Function: Transport dietary lipids to peripheral tissues.
Composition:
82% triacylglycerols
Key apoprotein: Apo B‑48
MTP (MTTP) - Microsomal triglyceride transfer protein : Facilitates lipid binding to Apo B‑48
Maturation: Acquire Apo C (I, II, III) and Apo E from HDL (storage)
Release: Exocytosed into lymph → bloodstream
Note:
MTP deficiency → abetalipoproteinemia (no “B” type protein)
Autosomal recessive
Low/absent chylomicrons
Severe neuropathy, acanthocytosis, steatorrhea in infancy
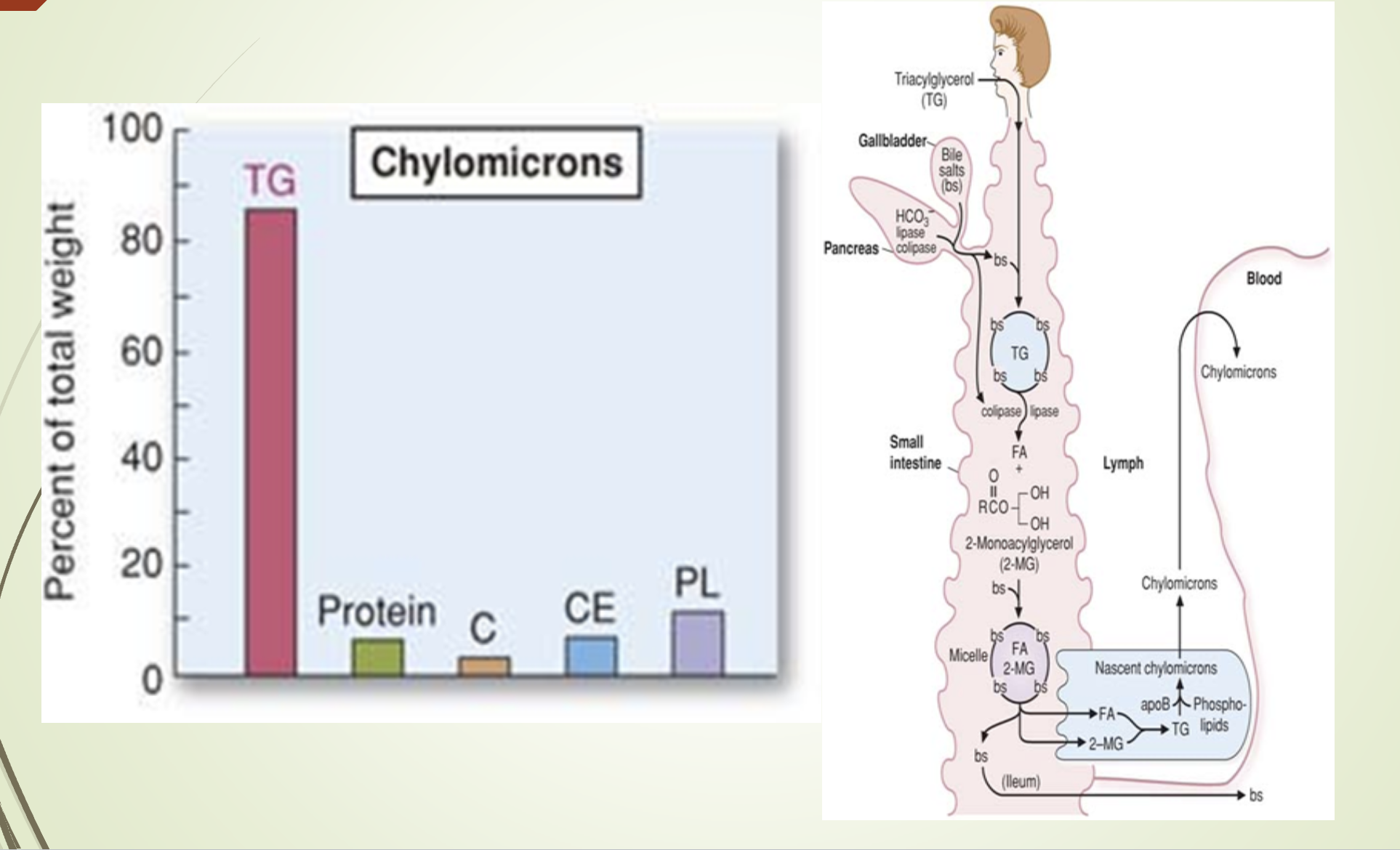
What does the chylomicron composition/synthesis slide emphasize?
Chylomicrons are synthesized in intestinal epithelial cells.
Composition dominated by triglycerides.
Structural apoprotein: Apo B‑48.
What apoprotein activates LPL and what are the structural proteins of VLDL/LDL vs chylomicrons?
Apo CII activates lipoprotein lipase (LPL).
Structural protein of VLDL & LDL: Apo B‑100
Structural protein of chylomicrons: Apo B‑48
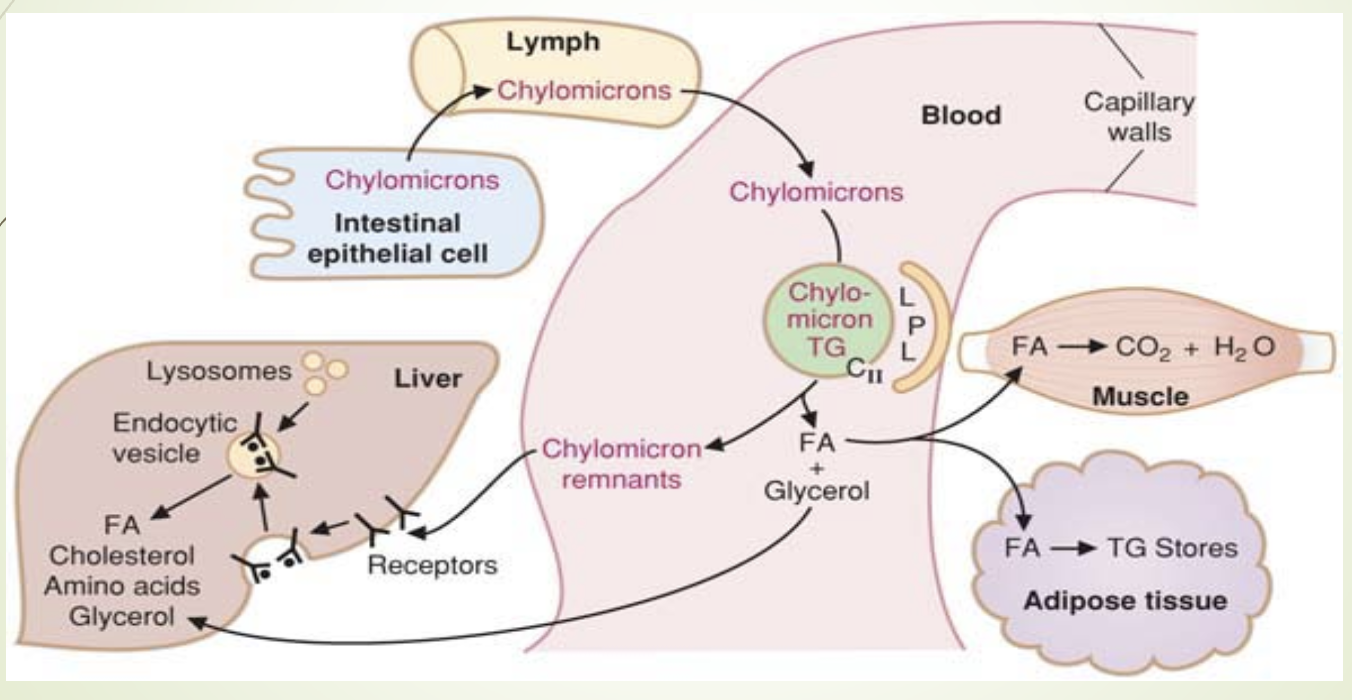
What is the fate of triglycerides in chylomicrons and where is LPL located?
TGs are hydrolyzed by LPL → fatty acids + glycerol
LPL is located on luminal surface of capillaries in:
Muscle (especially cardiac)
Adipose tissue
Lactating mammary gland
Lungs
Kidneys
Liver - last, doesn’t process full chylomicrons
FFAs bind albumin for transport
FFAs: oxidized for ATP (muscle) or stored as TG ie esterfied (adipose)
What regulates LPL and what happens in diabetes or LPL deficiency?
Adipose LPL has highest Km; heart LPL lowest Km
LPL synthesis stimulated by insulin
Low insulin → accumulation of chylomicrons & VLDL
Insulin inhibits hormone‑sensitive lipase
Diabetes mellitus:
Low insulin → low LPL + high HSL → hypertriglyceridemia
Familial LPL deficiency (Type I hyperlipoproteinemia):
Autosomal recessive
Severe hypertriglyceridemia (>2000 mg/dL)
What happens to glycerol released from triglycerides during LPL action?
Glycerol is transported to the liver.
In the liver, glycerol is phosphorylated → glycerol‑3‑phosphate.
Glycerol‑3‑phosphate is used for:
Triglyceride synthesis in the fed state
Gluconeogenesis in the fasting state
Blood glycerol increases when TGs are hydrolyzed by LPL.
What is the fate of chylomicron remnants and what receptors are involved?
Remnants contain:
Cholesteryl esters
Phospholipids
Apolipoproteins
Small amount of TG
Remnants bind hepatocyte receptors via Apo E:
LDL receptor–related protein (LRP)
Remnants contain equal cholesterol and triglycerides.
Endocytosed and hydrolyzed in lysosomes.
How are chylomicron remnants processed after ApoE recognition?
ApoE binds LRP on hepatocytes.
Endocytosis → lysosomal degradation.
Products released into cytosol:
Fatty acids/Amino acids/Glycerol/Cholesterol
Type III hyperlipoproteinemia (dysbetalipoproteinemia):
Defective remnant removal
Autosomal recessive
Premature atherosclerosis
Xanthoma striata palmaris (palmar xanthomas)
Which lipidemia is more severe: ApoCII deficiency or ApoE deficiency?
ApoCII deficiency causes more severe lipidemia.
Because ApoCII is required to activate LPL, so TGs cannot be hydrolyzed at all. —> more severe
ApoE deficiency affects remnant clearance but does not block TG hydrolysis.
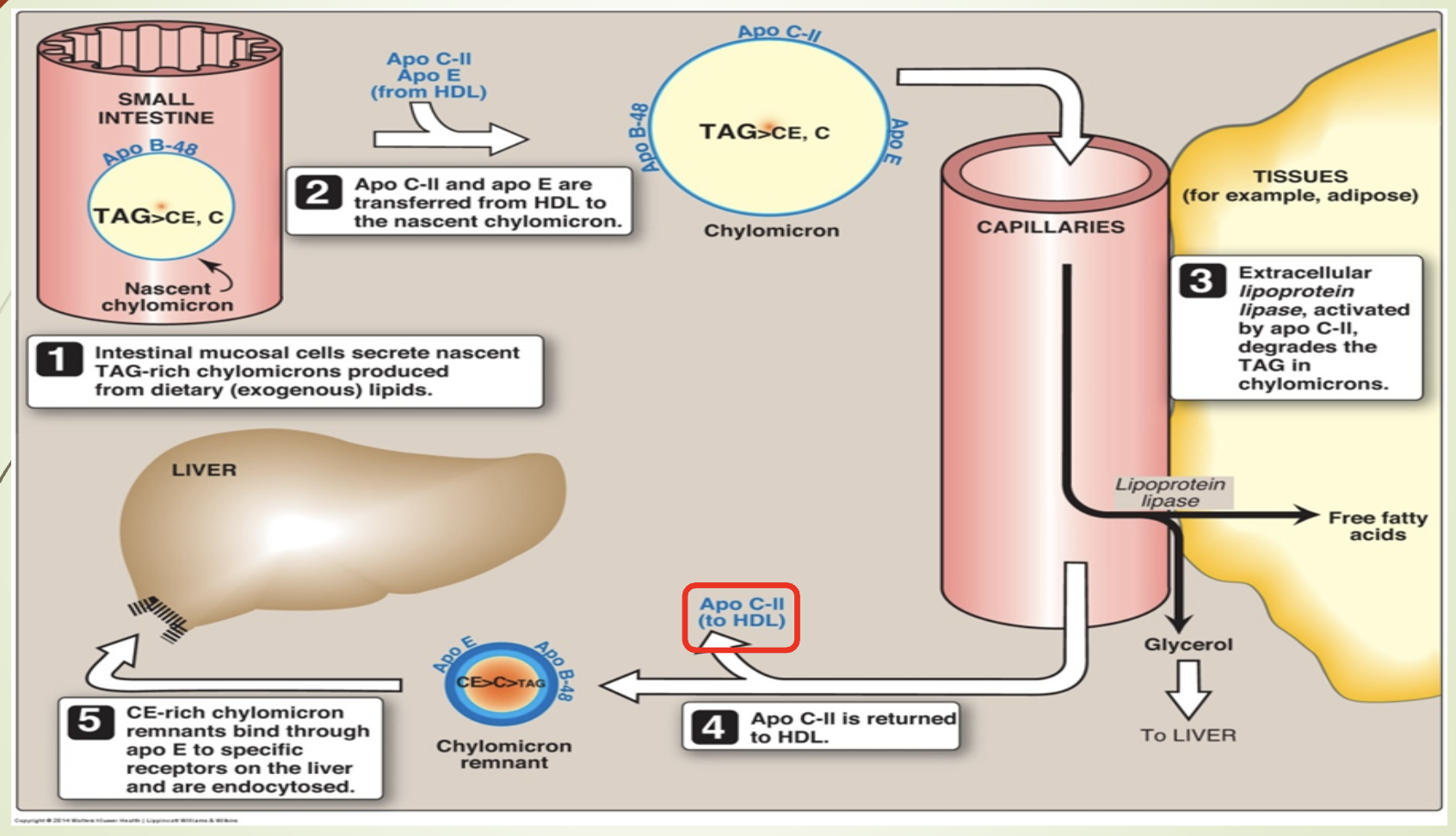
What does the chylomicron metabolism slide emphasize?
Synthesis in intestine → lymph → blood.
Maturation via ApoC and ApoE from HDL.
LPL (activated by ApoCII —> goes back to HDL) hydrolyzes TGs.
Remnants taken up by liver via ApoE‑LRP.
What is the composition, synthesis, and function of VLDL?
Composition:
52% TG (Hepatic aka endogenous)
22% cholesterol
18% phospholipids
8% apoproteins
Synthesis:
From chylomicron remnants
From TAGs not hydrolyzed in peripheral tissues
From dietary carbohydrates (major carbon source):
Glucose → acetyl‑CoA → fatty acids
Glucose → DHAP → glycerol
FA + glycerol → TAG
High‑carb intake → carbohydrate‑induced hypertriglyceridemia
Major apoprotein: Apo B‑100
Acquire ApoC and ApoE from HDL
MTTP loads TG onto ApoB100
Function: Transport hepatic lipids to peripheral tissues.
What is lipogenesis and where does it occur?
Lipogenesis = synthesis of TGs from glucose.
Occurs in the liver.
Fatty acids synthesized from glucose → converted to TGs → packaged into VLDL → secreted into blood.
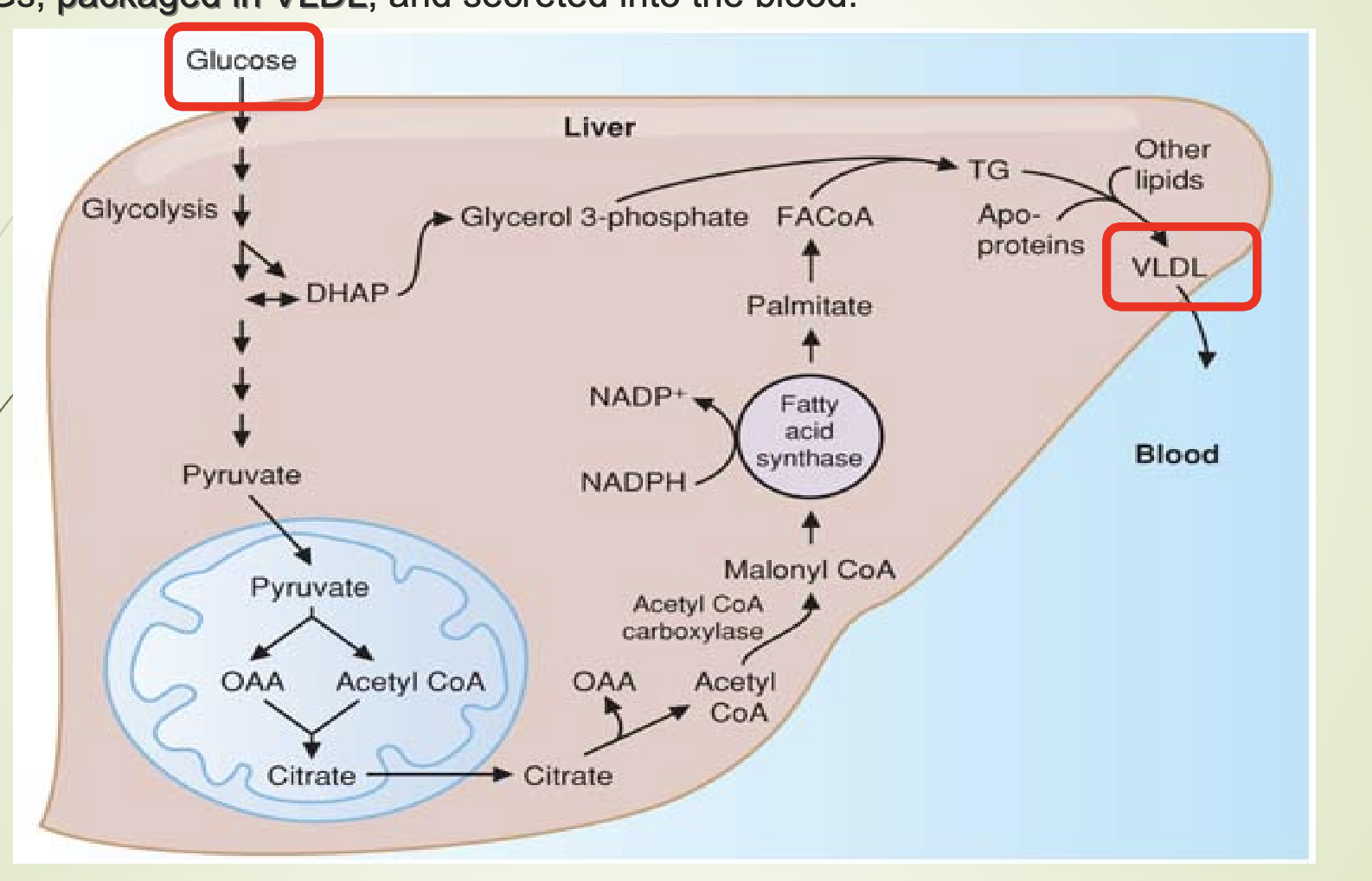
What happens when VLDL production exceeds secretion?
VLDL transports TG from liver to tissues.
If liver TG > VLDL secretion → fatty liver (hepatic steatosis).
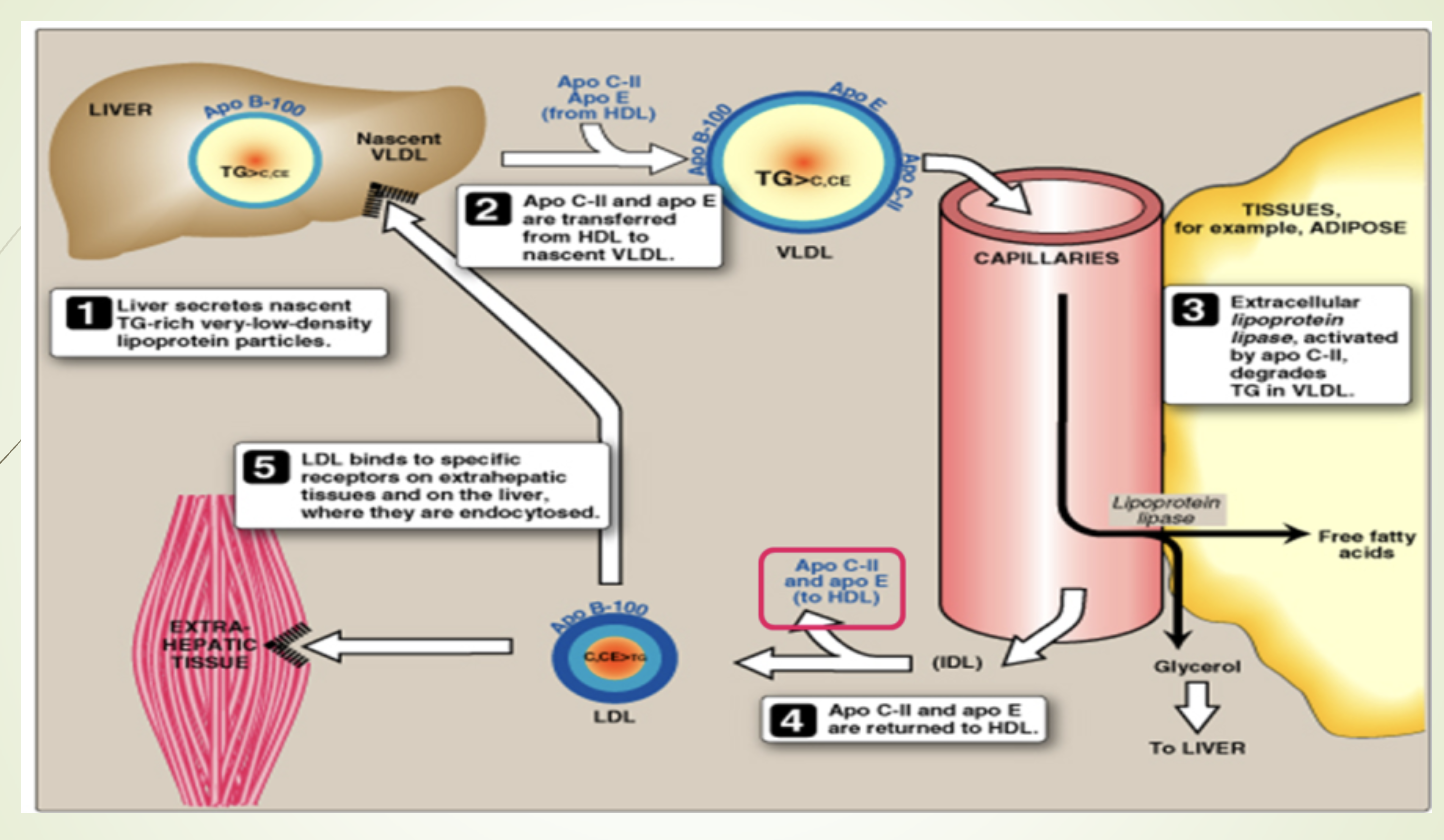
What happens to VLDL after LPL hydrolysis?
ApoC returned to HDL.
VLDL remnants can:
Be internalized by liver, or
Be converted to IDL → LDL
Conversion IDL → LDL requires hepatic triglyceride lipase (HTGL).
What modifications occur as VLDL becomes IDL and LDL?
LPL removes TG → particle becomes smaller and denser.
ApoCII and ApoE returned to HDL.
TG exchanged for cholesteryl esters via CETP.
CETP deficiency → high HDL, low LDL, low CHD incidence.
Cholesteryl ester transfer protein (CETP)
What happens to VLDL triglycerides and what are the possible fates of IDL and LDL?
VLDL TGs are hydrolyzed by LPL → fatty acids + glycerol.
Fatty acids:
Oxidized for energy (muscle)
Stored as TG (adipose)
Glycerol: used by liver and tissues with glycerol kinase.
VLDL → IDL → LDL via HTGL.
LDL can be:
Endocytosed by liver
Endocytosed by peripheral cells
Oxidized and taken up by macrophage scavenger receptors → atherosclerosis
HTGL deficiency:
Low LDL
High TG
High cholesterol
Accumulation of VLDL + chylomicron remnants
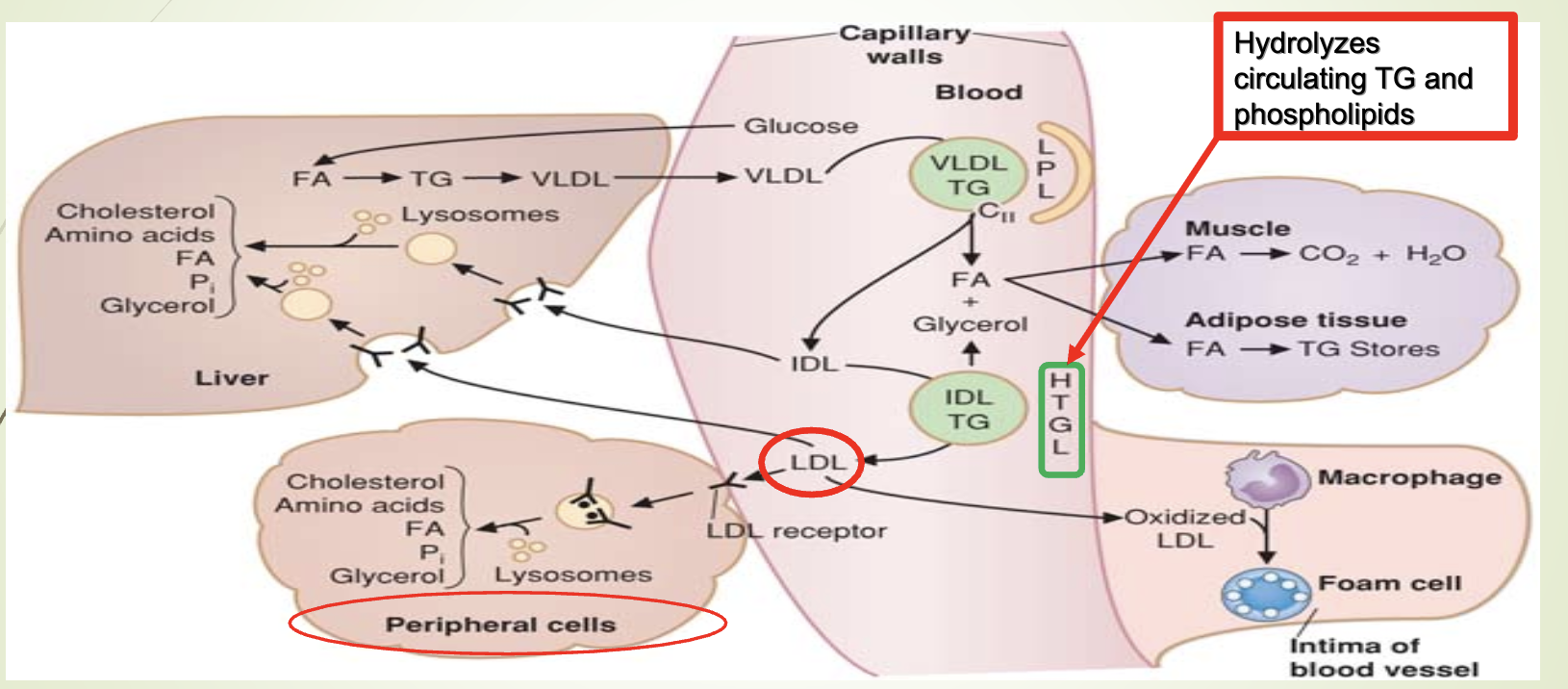
How are fatty acids from chylomicrons and VLDL converted into stored triglycerides in adipose tissue?
LPL releases fatty acids from TG in chylomicrons/VLDL.
Fatty acids enter adipocytes.
Re‑esterified with glycerol‑3‑phosphate to form TG.
Stored in adipose cells.
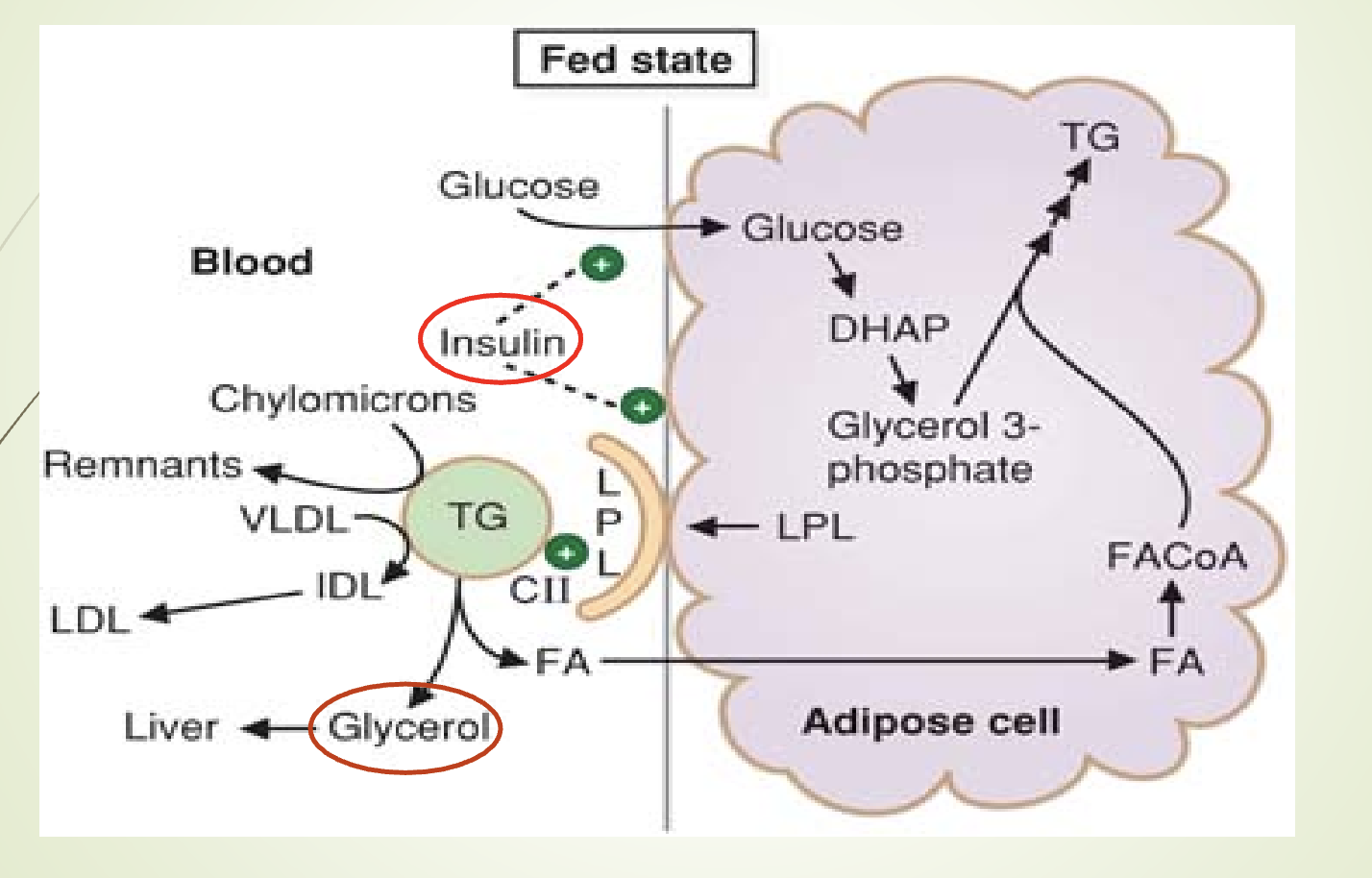
What is the composition, synthesis, and function of LDL?
Composition:
47% cholesterol
23% phospholipids
21% apoprotein (only Apo B‑100)
9% triglycerides
Synthesis:
Formed from VLDL catabolism: VLDL → IDL → LDL
Function:
Transports ≈60% of total body cholesterol (fasting plasma).
2/3 of LDL cholesterol is esterified (linoleic acid).
Major carrier of cholesterol to peripheral tissues.
What enzyme esterifies cholesterol in plasma and what disease results from its deficiency?
LCAT (lecithin:cholesterol acyltransferase) esterifies cholesterol in plasma.
Familial LCAT deficiency:
Complete absence of LCAT activity
Esterification defects in HDL and LDL
Triad:
Diffuse corneal opacities
Target cell hemolytic anemia
Proteinuria with renal failure
Question posed: etiologies of target cells include liver disease, hemoglobinopathies, and LCAT deficiency.
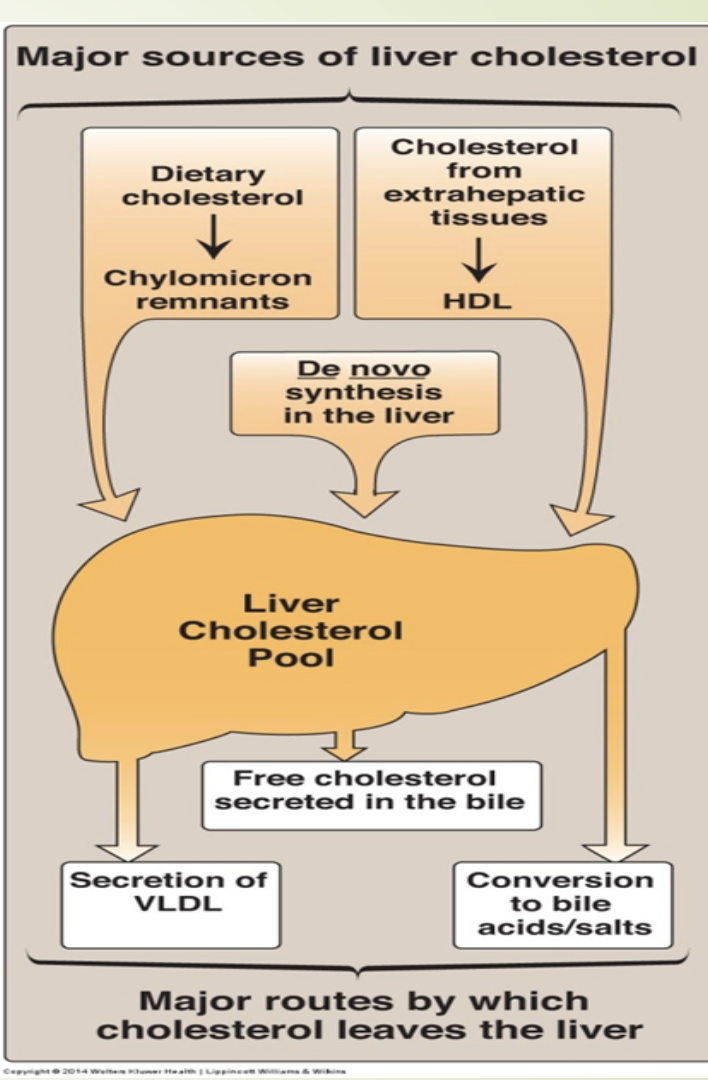
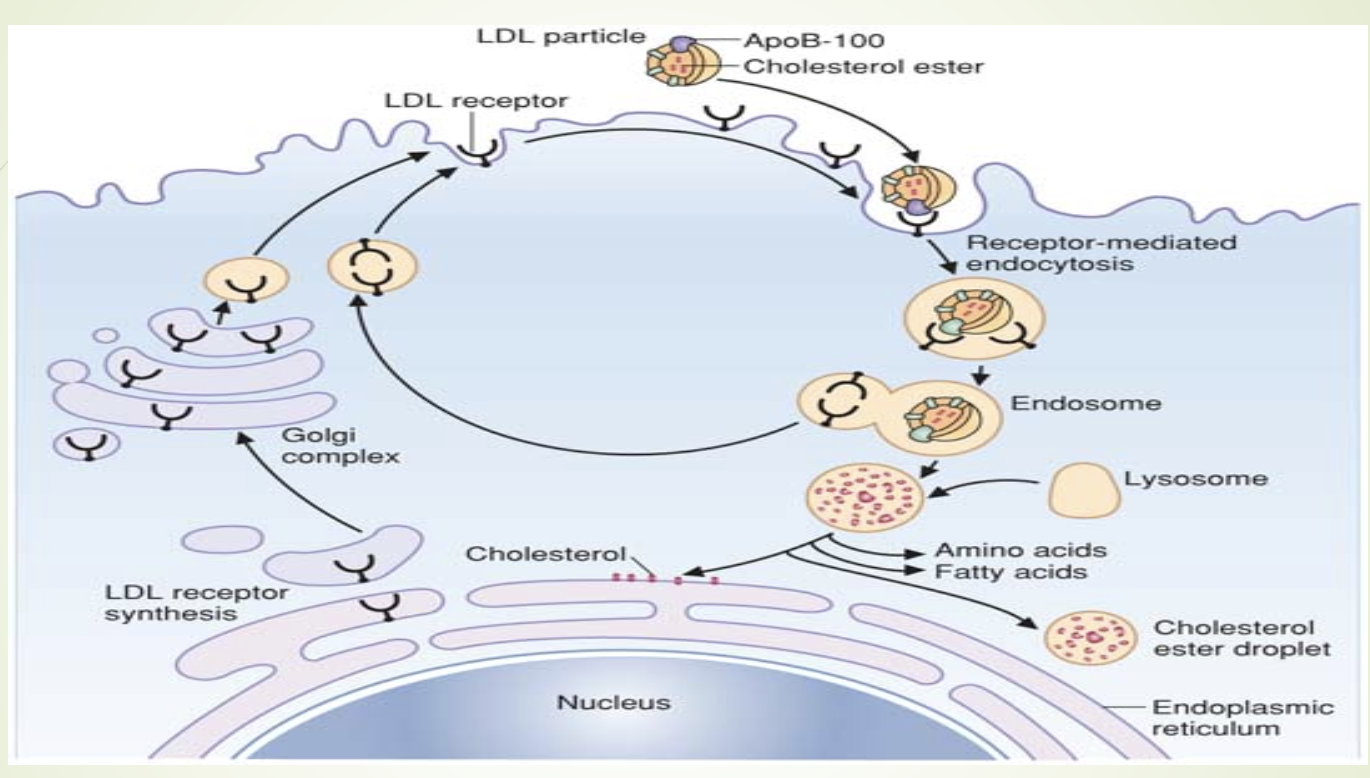
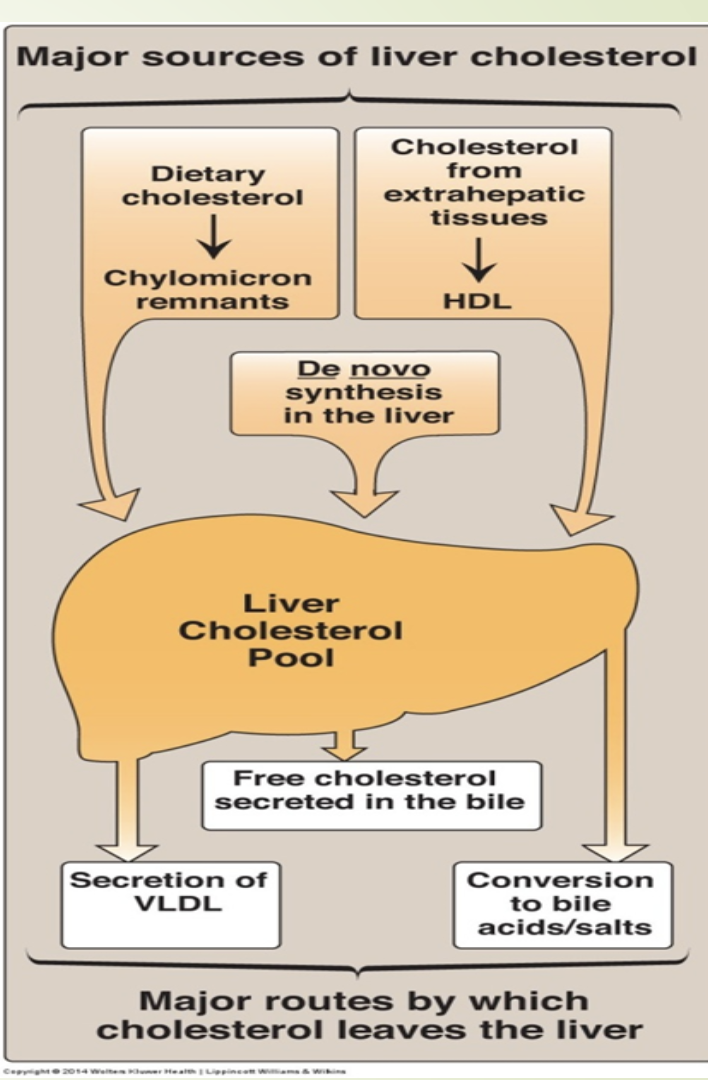
How is LDL cleared and how does free cholesterol regulate hepatic cholesterol metabolism?
LDL taken up by hepatocytes via LDL receptors.
Lysosomal degradation →
Free cholesterolesterified by acyl-CoA cholesterol acyl
transferase (ACAT)
Amino acids
Increased free cholesterol causes:
↓ HMG‑CoA reductase activity
↑ ACAT activity
↓ LDL receptor production
Note:
LDL receptor deficiency → Type II hyperlipidemia (familial hypercholesterolemia) → premature atherosclerosis.
What hormone affects LDL receptor binding and what condition results from its deficiency?
Thyroid hormone T3 stimulates LDL receptor binding.
Hypothyroidism → decreased LDL receptor activity → hypercholesterolemia secondary to hypothroidism
What actions can decrease total cholesterol in the bloodstream? LY
Examples include:
Lifestyle:
Diet low in saturated fat
Increased physical activity
Weight loss
Smoking cessation
Pharmaceutical:
Statins (HMG‑CoA reductase inhibitors)
Bile acid sequestrants
Ezetimibe
PCSK9 inhibitors
What is Lp(a), what risks does it pose, and what are its characteristics?
Lp(a) resembles LDL.
Increases risk of CVD.
More atherogenic than LDL.
Promotes clot formation (interferes with fibrinolysis).
Genetically determined.
Synthesized in liver; contains Apo B‑100.
Some patients benefit from lipoprotein apheresis (↓ Lp(a) by 75%).
Secondary causes: CKD, nephrotic syndrome, hypothyroidism.
What is the composition, synthesis, apoproteins, and function of HDL?
Composition:
50% apoproteins
28% phospholipids
19% cholesterol
3% triglycerides
Synthesis:
Assembled in liver and intestine from peripheral tissue
Major apoproteins:
Apo A‑I
Apo A‑II
Function:
Reverse cholesterol transport (tissues → liver)
Free cholesterol esterified by LCAT on HDL (Cholesterol + FA → cholesterol ester)
Question posed: Predict consequences of absence of Apo A → extremely low HDL, impaired reverse cholesterol transport, ↑ CHD risk.
How does HDL deliver cholesterol to the liver and interact with other particles?
HDL binds hepatocytes via SR‑B1.
Releases cholesterol esters without endocytosis.
SR‑B1 upregulated when cholesterol demand is high.
HDL exchanges ApoC and ApoE with chylomicrons, VLDL, and IDL.
SR‑B1 is multifunctional
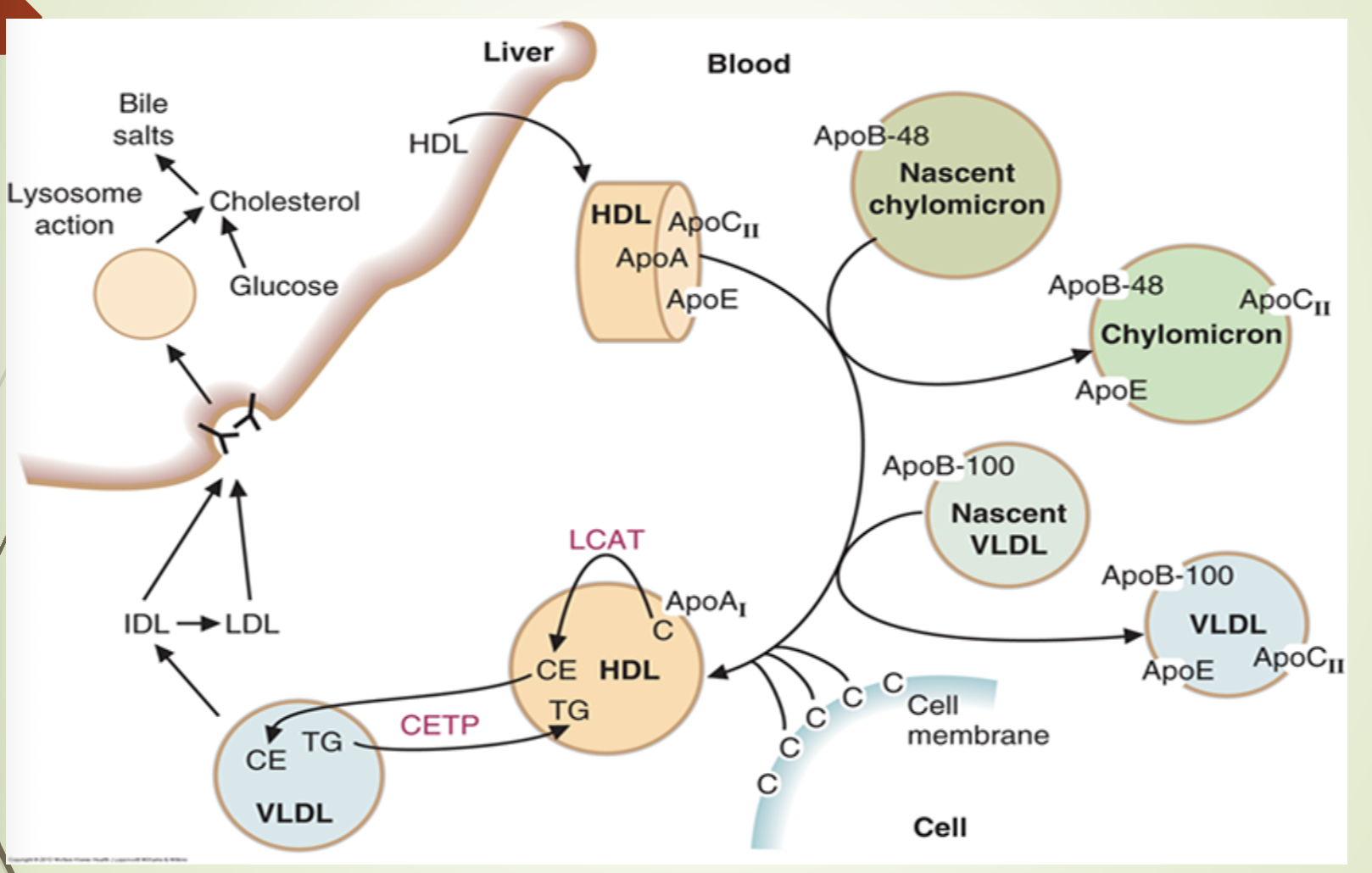
What does the recap slide emphasize about lipoprotein metabolism?
It summarizes the pathways of chylomicrons, VLDL, IDL, LDL, and HDL.
Reinforces the roles of apoproteins, LPL, HTGL, CETP, and receptor‑mediated uptake.
Highlights the flow of dietary and hepatic lipids through the bloodstream and into tissues or liver.
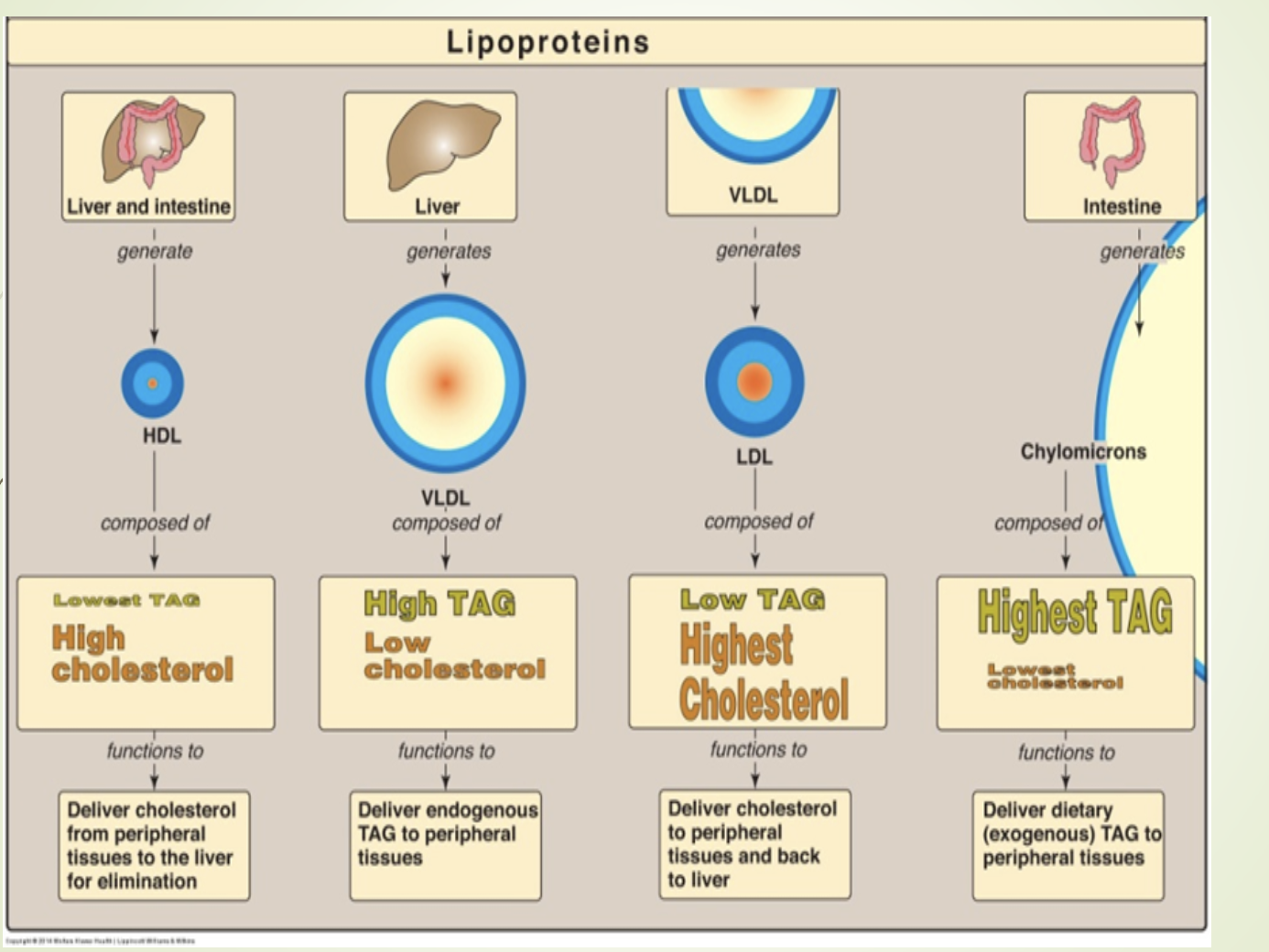
What is the overall summary of lipoprotein metabolism presented on this slide?
Chylomicrons transport dietary TG.
VLDL transports hepatic TG.
IDL and LDL arise from VLDL metabolism.
LDL delivers cholesterol to tissues.
HDL removes cholesterol from tissues and returns it to the liver.
Apoproteins regulate enzyme activation, receptor binding, and particle stability.
KEY WORD: MILKY APPEARANCE (after 12h fasting)
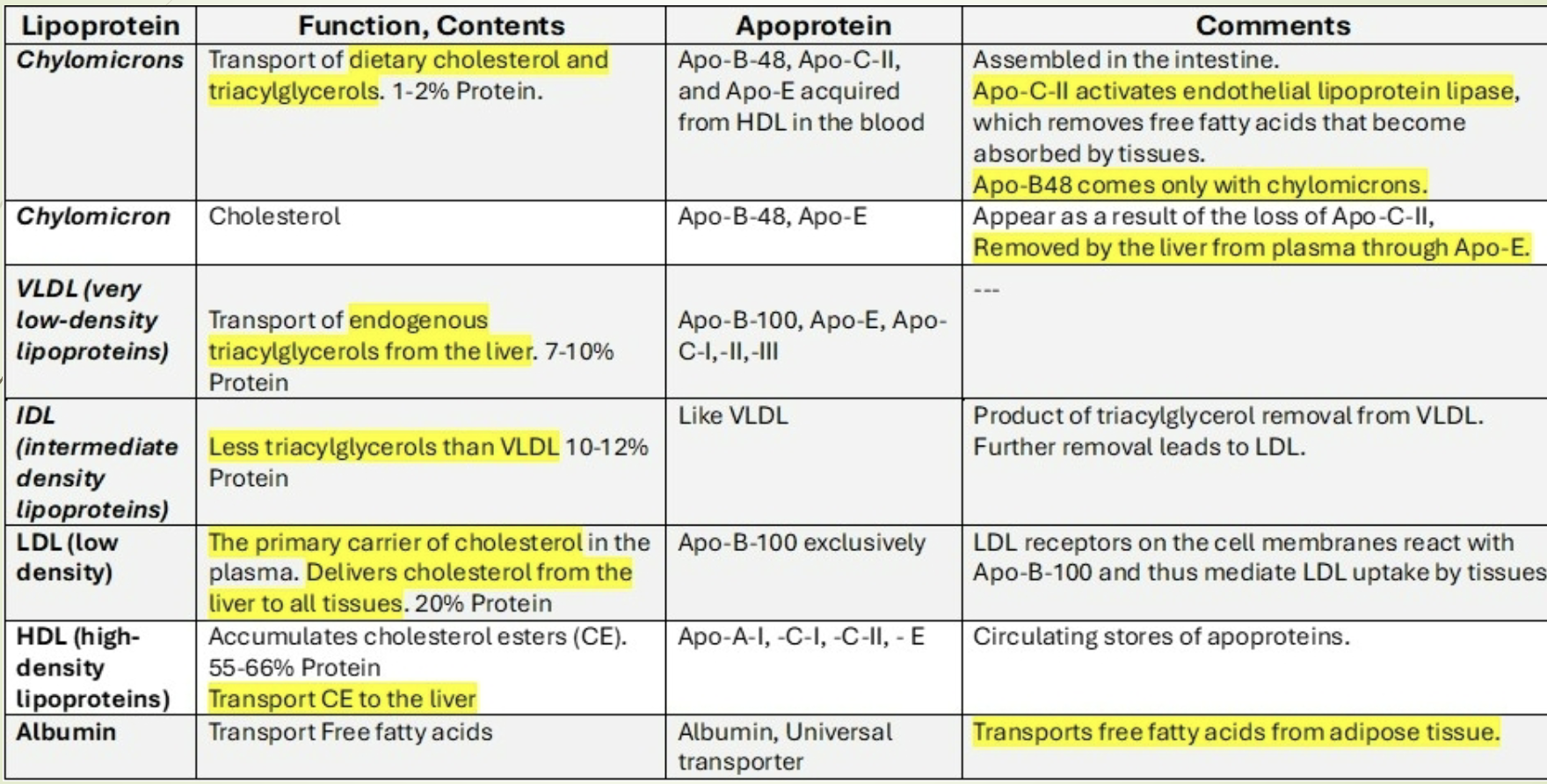
What are the major apoproteins and their key characteristics? Review
Apo A‑I: Activates LCAT; major HDL protein.
Apo A‑II: HDL structural protein.
Apo B‑48: Structural protein of chylomicrons.
Apo B‑100: Structural protein of VLDL, IDL, LDL; binds LDL receptor.
Apo C‑II: Activates LPL.
Apo C‑III: Inhibits LPL.
Apo E: Required for remnant uptake by liver (chylomicron remnants, IDL).
What are the major lipid fractions in fasting serum and how is total cholesterol calculated?
Cholesterol carried on VLDL, LDL, HDL.
Total cholesterol (TC) = HDL + VLDL + LDL.
Clinical labs measure:
Total cholesterol/ triglycerides/HDL cholesterol
In fasting serum, most TG are in VLDL
VLDL cholesterol ≈ TG/5.
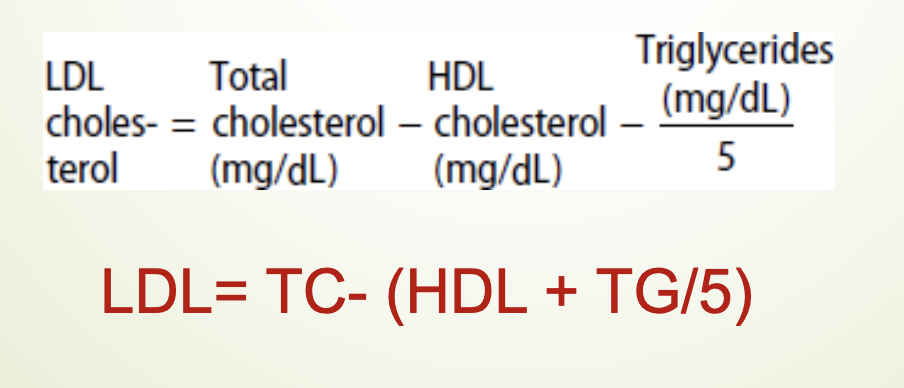
What is the Friedewald formula and when is it valid?
LDL = TC – (HDL + TG/5)
Valid only when:
Fasting sample
TG < 400 mg/dL
If TG > 400 mg/dL → need ultracentrifugation or direct LDL measurement.
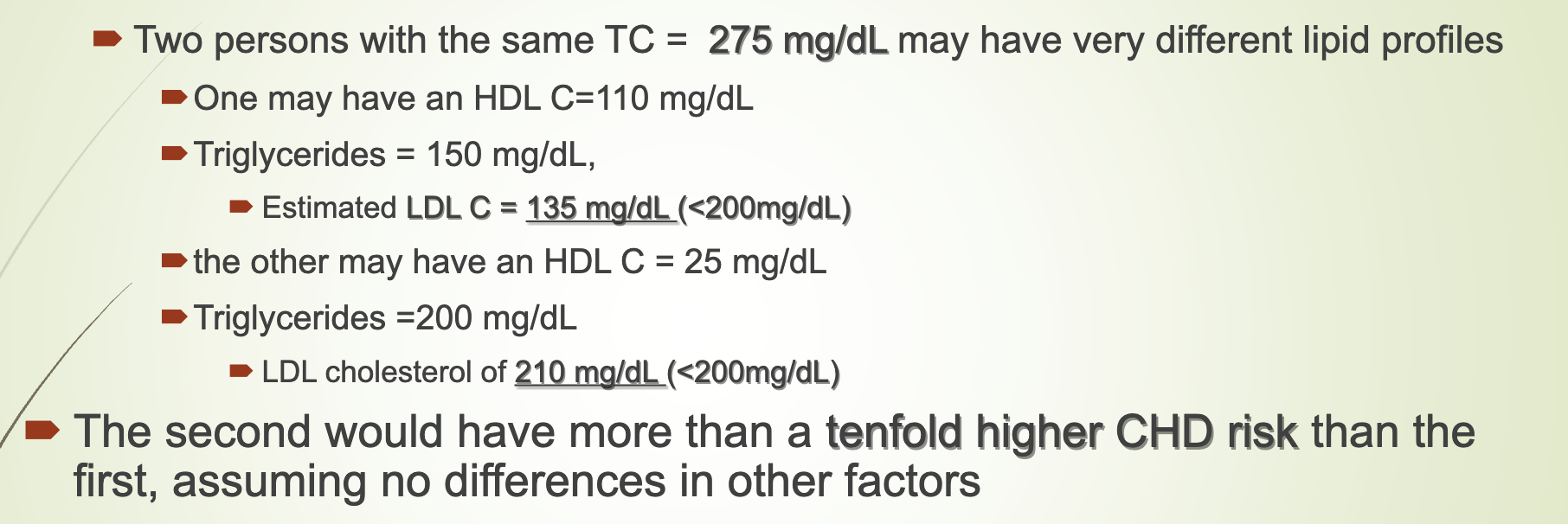
Why is evaluating lipid fractions more accurate than total cholesterol alone?
Two people with same TC can have vastly different LDL, HDL, and TG.
Example:
High HDL → lower CHD risk
Low HDL + high LDL → much higher CHD risk
Women often have high HDL (activity dep), making TC misleading.
Lipid fractions must be evaluated before therapy.
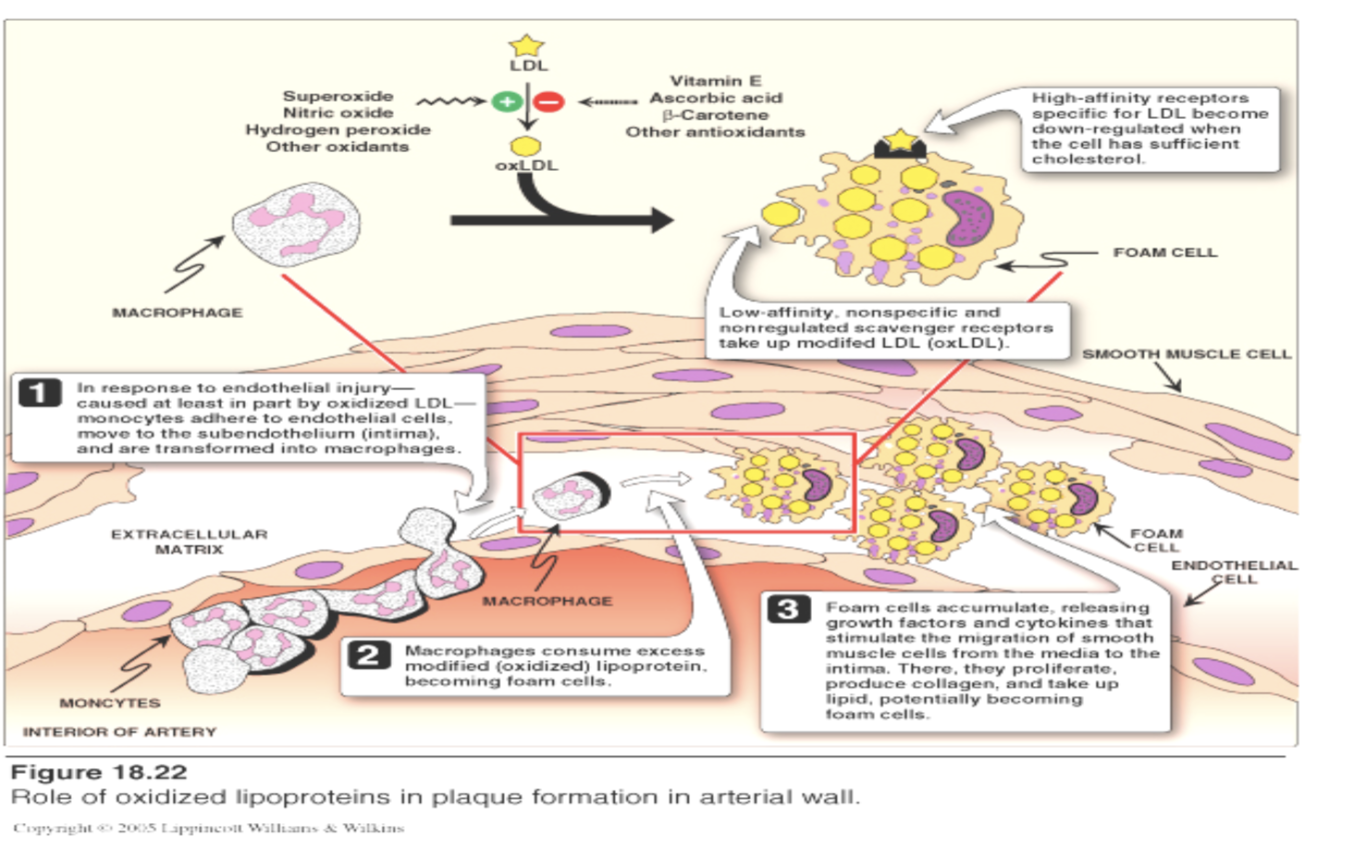
How do macrophage scavenger receptors contribute to plaque formation?
Macrophages take up oxidized LDL via scavenger receptors.
Uptake is unregulated → foam cell formation.
Foam cells accumulate in arterial intima → atherosclerotic plaque.
What are the positive and negative risk factors for CHD? LY
Positive risk factors:
Age:
Male ≥ 45
Female ≥ 55 or premature menopause without estrogen therapy
Family history of premature CHD
Current smoking
Hypertension
Diabetes mellitus
Low HDL (< 35 mg/dL)
High Lp(a)
Negative risk factor:
High HDL (≥ 60 mg/dL)
What secondary conditions can alter lipid levels and why are they important?
Important because:
Abnormal lipids may be the first sign of an underlying condition.
Treating the underlying condition may eliminate the lipid disorder.
Diabetes and alcohol use commonly cause high TG.
Improving glycemic control or reducing alcohol lowers TG.
Secondary causes should be evaluated before starting lipid‑lowering therapy.
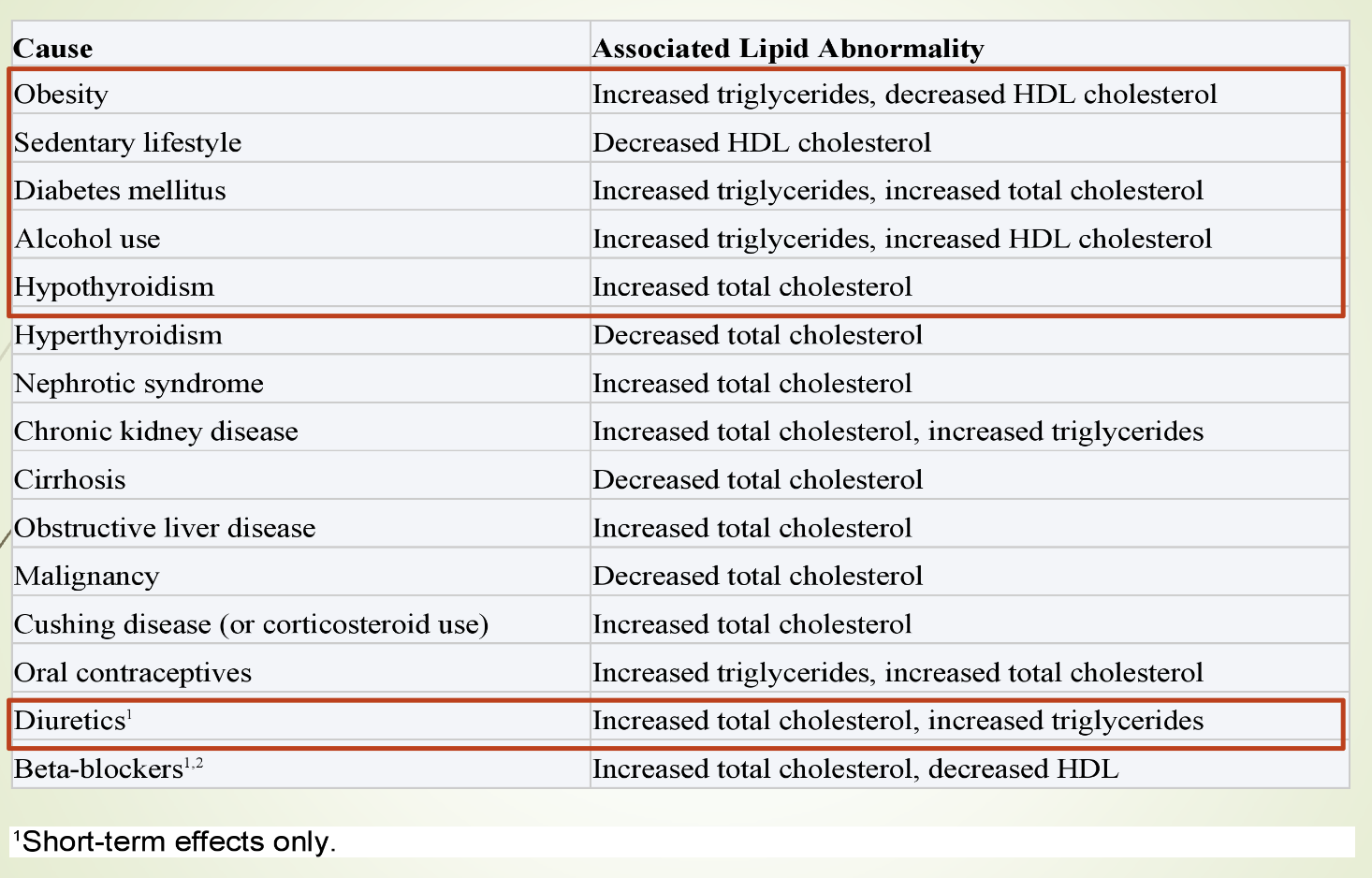
What does eTable 28–1 summarize regarding lipid abnormalities?
Lists secondary causes of lipid abnormalities from Current Medical Diagnosis & Treatment.
Includes conditions, medications, and metabolic disorders that alter lipid levels.
Emphasizes that secondary causes must be evaluated before diagnosing primary lipid disorders.
What additional risk factors help predict future CHD events?
High‑sensitivity C‑reactive protein (hs‑CRP)
Electron beam computed tomography (EBCT)
Homocysteine
Fibrinogen
Lipoprotein (a)
LDL particle characteristics
Treatment decisions are based on:
Presence of clinical cardiovascular disease or diabetes
Patient age
LDL cholesterol > 190 mg/dL
Estimated 10‑year cardiovascular risk
Increased fiber = increased bile excretion —> lowers cholesterol in blood from making more bile
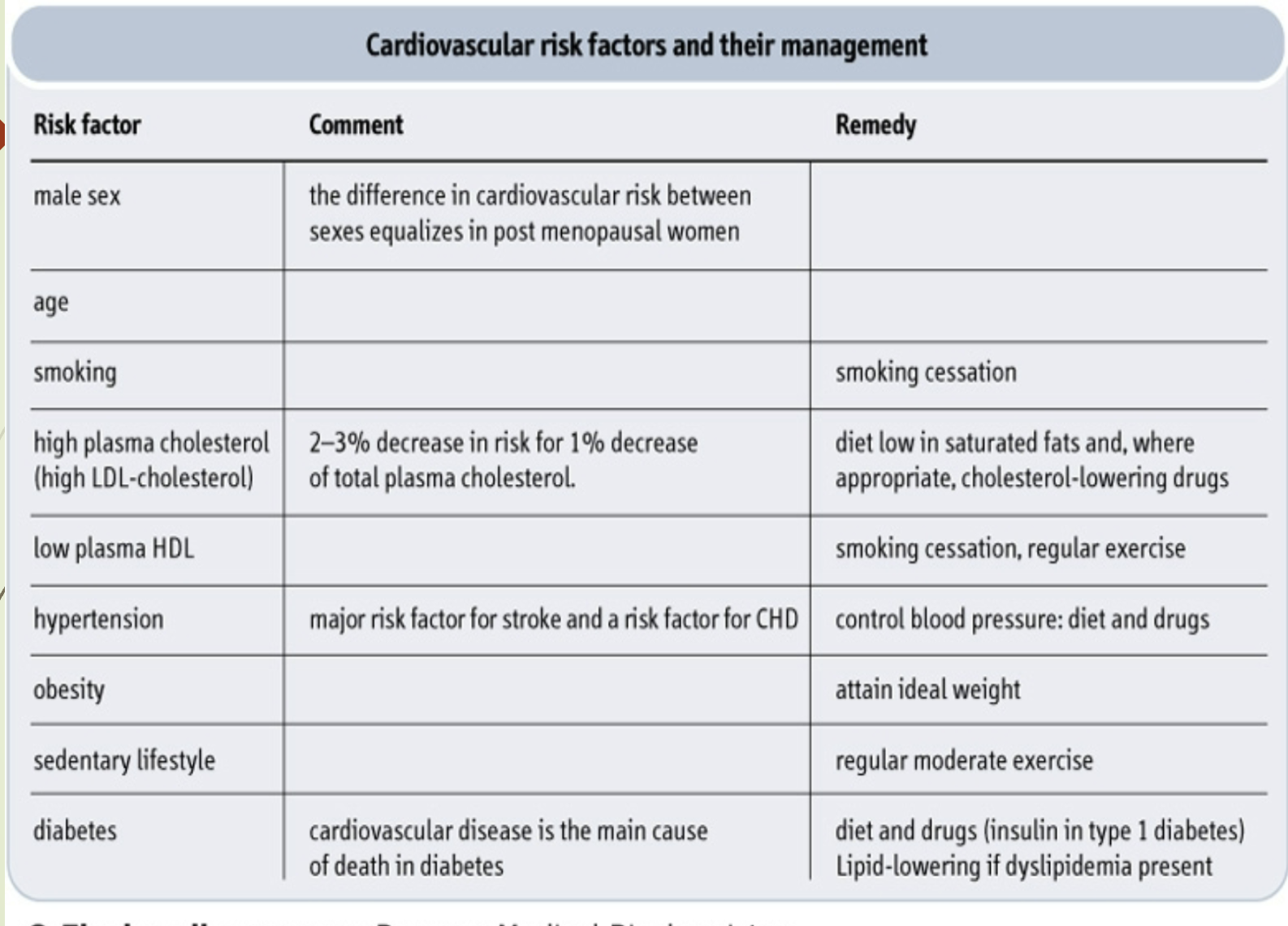
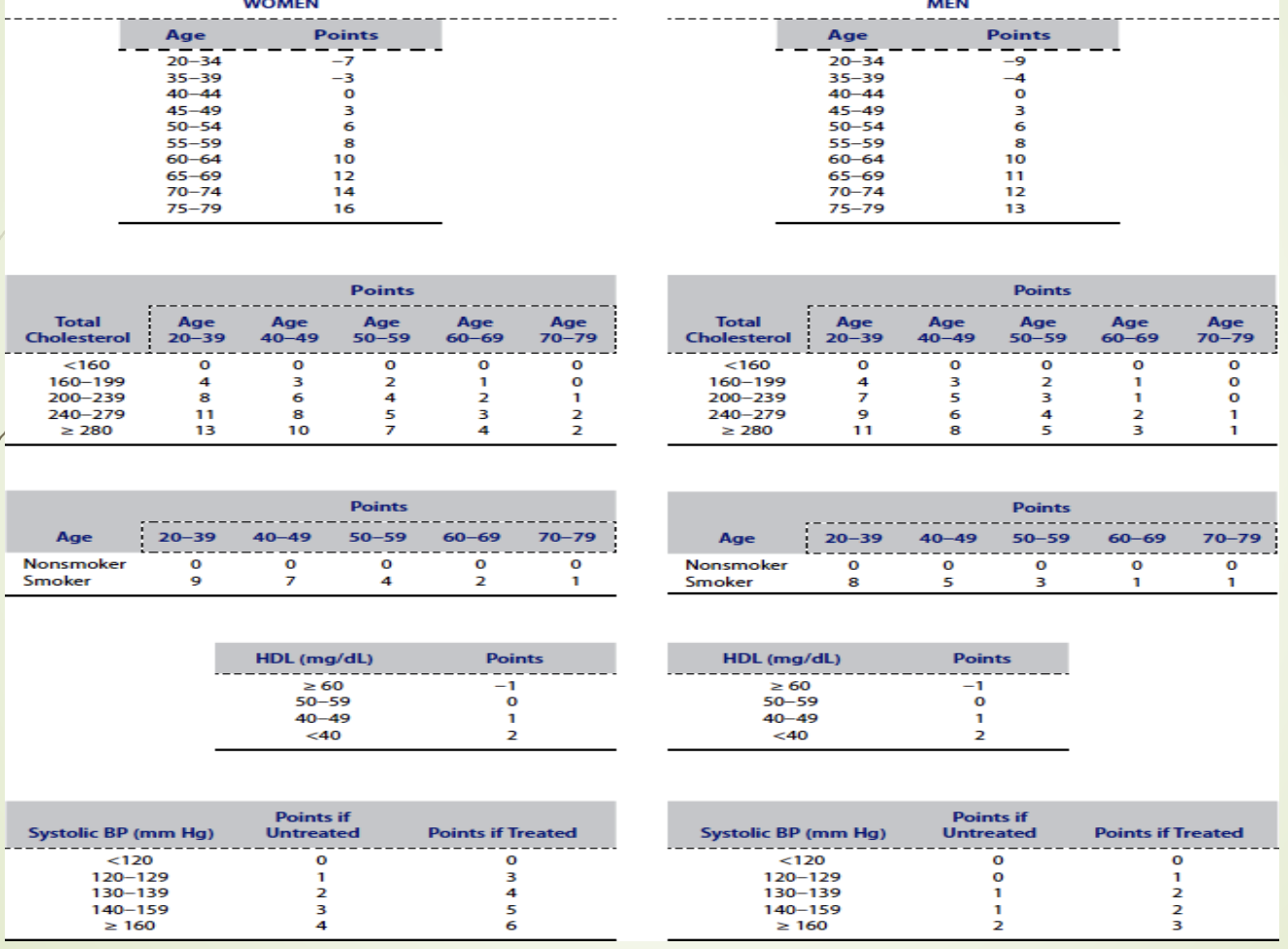
What is the purpose of the Framingham 10‑year CHD risk table? LY
Assigns point values to risk factors.
Total score estimates 10‑year CHD risk.
Used to guide treatment decisions and preventive strategies.
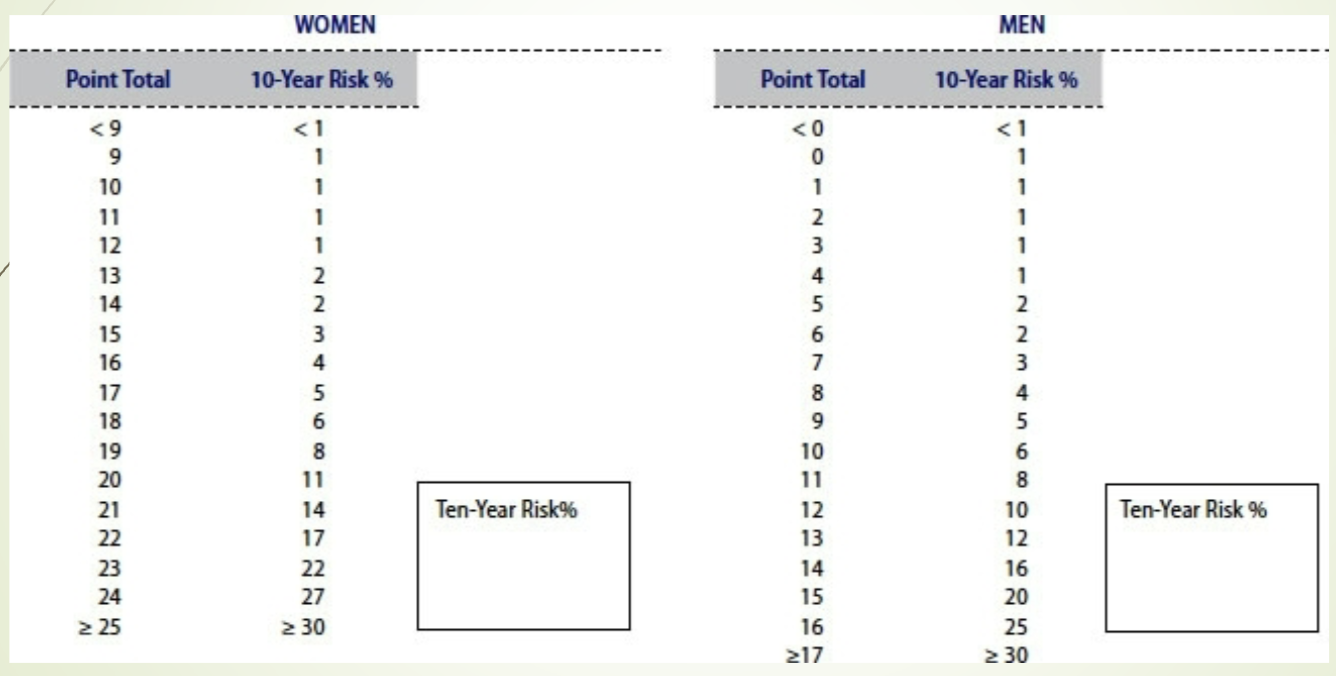
What does the 10‑year risk estimation slide provide? LY
Converts total Framingham points into a percentage risk of developing CHD within 10 years.
Used clinically to stratify patients into low, intermediate, or high risk.
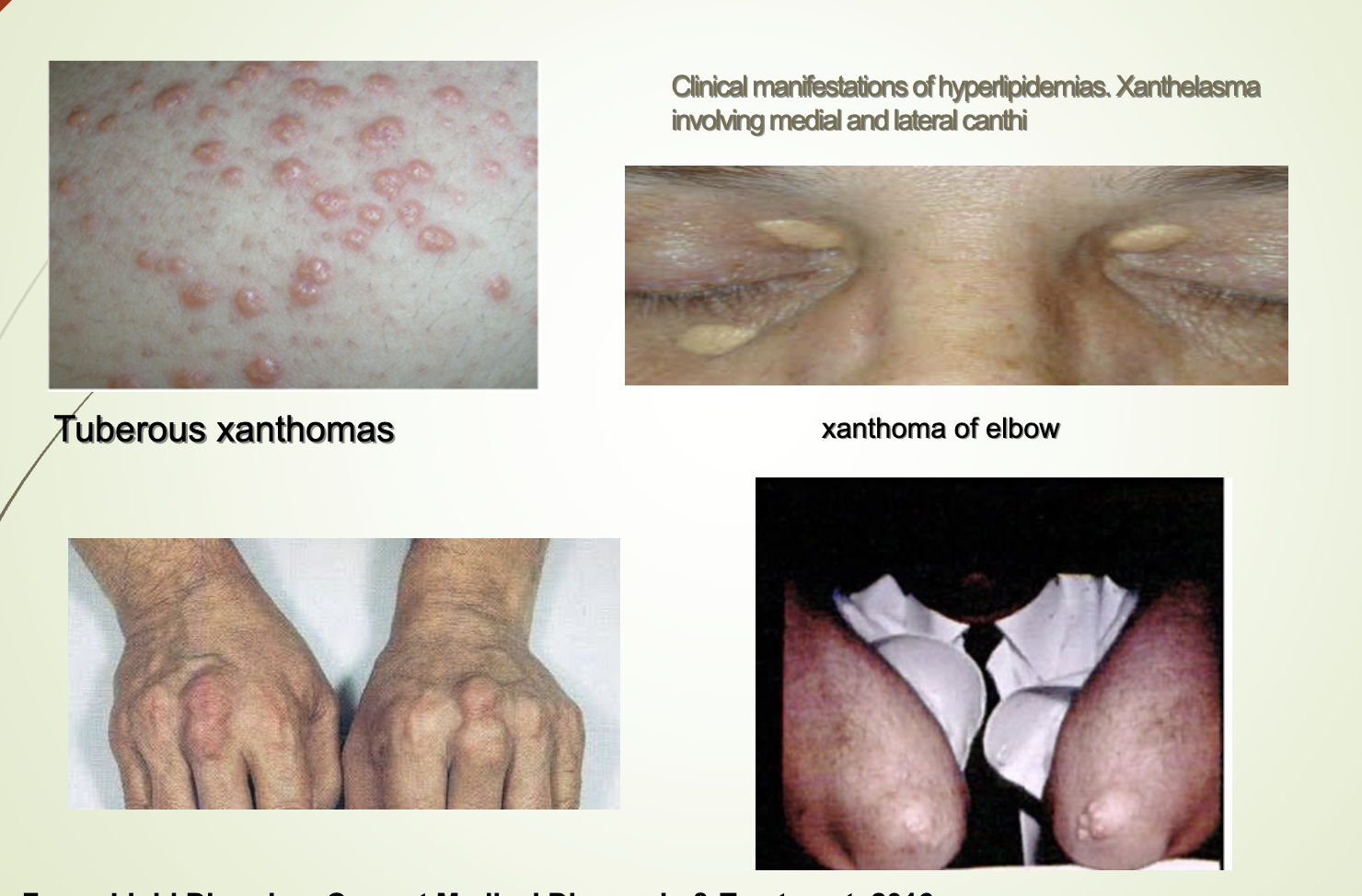
What are the clinical presentations associated with lipid abnormalities?
Most patients have no symptoms.
Detected via lab testing during CVD workup or preventive screening.
Extremely high chylomicrons or VLDL (TG > 1000 mg/dL): eruptive xanthomas.
High LDL: tendinous xanthomas (Achilles, patella, hand).
Tendon xanthomas suggest genetic hyperlipidemias.
Familial combined hyperlipidemia (FCHL):
Most common inherited dyslipidemia
Elevated LDL‑C + TG
Variable phenotypes
Strong family history of premature ASCVD
Tendon xanthomas absent (distinguishes from FH)
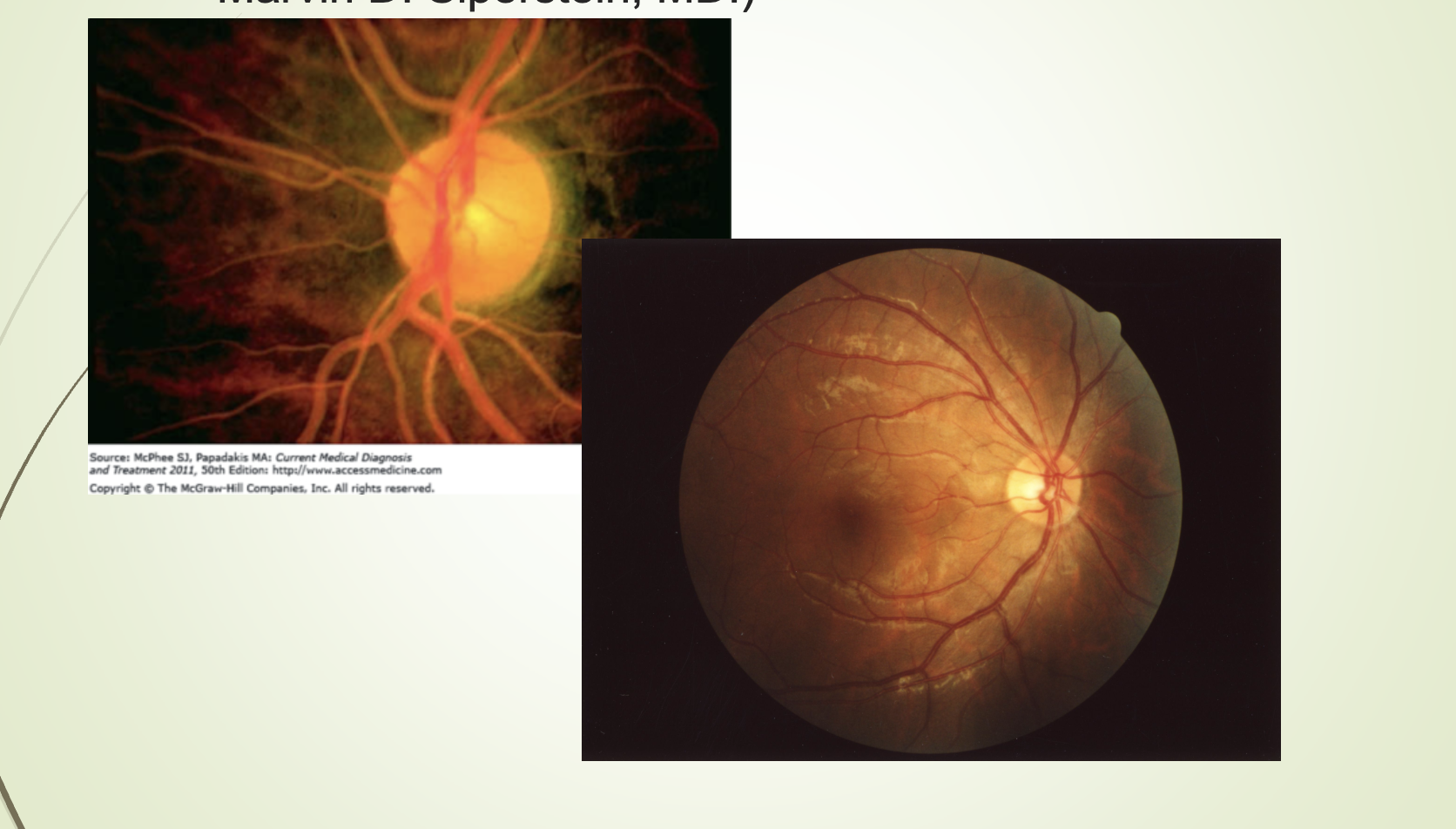
Lipemia retinalis: TG > 2000 mg/dL → cream‑colored retinal vessels.
What condition is shown in the lipemia retinalis image and what causes it?
Lipemia retinalis.
Caused by extremely high triglycerides (> 2000 mg/dL).
Retinal vessels appear cream‑colored.
What clinical findings are shown in the eruptive xanthoma?
Eruptive xanthomas on the arm in untreated hyperlipidemia + diabetes mellitus.
Xanthelasma on eyelids.
Tuberous xanthomas.
Elbow xanthomas.
All associated with severe hyperlipidemia.