Chemistry
History and evolution of the Atom.
Matter is composed of atoms, which are the basic building blocks and the smallest units of matter, capable of forming molecules through chemical bonds.
Energy isn’t considered Matter.
Energy can impact matter and matter can impact energy.
Atom is the building block of matter
John Dalton: Said all atoms were indivisible and indestructible, establishing the foundation for atomic theory and the understanding of chemical reactions. We know now this is incorrect.
Pure substance: only one type of particle (ie. one type of compound or element). It must have a fixed composition and fixed properties.
Mixture: a combination of two or more particles which are not chemically bounded. It can be separated through physical means such as filtration.
Compound: a combination of two or more elements which are chemically bonded. Can be chemically separated.
Element: only one type of atom which are chemically bonded. Can’t be chemically or physically separated.
Atomic model
Protons are positive, Neutrons are neutral and electrons are negative.
Neutrons have the highest mass of a sub-atomic particle.
Electrons decide how reactive an element is. Each element is defined by its atomic number, which corresponds to the number of protons in its nucleus.
Protons have a charge of 1, neutrons have a charge of 0, electrons have a charge of -1.
The Bohr model is the most widely known atomic model, illustrating electrons orbiting the nucleus in defined energy levels.
Rutherford said electrons simply orbit the nucleus while Bohr said electrons have fixed orbits on shells which correspond to energy level.
The highest electron shell/energy level is 7. The energy levels increase as we move away from the nucleus.
The highest electron shell has the most energy as electrons need to spin and the closer the electron the less spin they get, leading to low energy in the internal shells. This happen as the electromagnetic pull of the nucleus.
Electrons that occupy the same energy level, will spin anti-clockwise and are referred to as having parallel spins, which contributes to the overall magnetic properties of the atom. However, electrons don’t like being alone.
All n1 shells will have the same energy level regardless of the element,
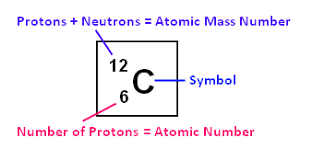
Atomic number is the number of protons in the nucleus. The number of protons= number of electrons
Atomic Mass Number is the number of Protons + Neutrons
All element are electrically nucleus, meaning the number of protons is equal to the number of neutrons.
Isotopes are variation of the same element. The only difference is the the number of neutrons. This changes the Atomic Mass of the element.
The only determinant of Atomic identity (the element’s name) is the number of protons.
The periodic table shows you the most abundant isotope of a element.
The number of neutrons does not impact the properties of the element.
Neutrons determine physical properties of an element (like mass and density)
Isotopes can become very heavy. If said isotope becomes too heavy, the nucleus becomes radioactive by ejecting neutrons to make itself lighter.
In organic matter, carbon is the most abundant element; especially carbon-14.
Each shell hold 2n² electrons, where n= shell number.
This only really works for elements 1-18.
A group on the periodic table is a column.
A period is a row on the periodic table.
Valence electrons are the outermost electrons and valence electron shell is the outer shell.
Periodic table
Groups are in rows. There are 7 groups, with each element in a period having the corresponding number of shells. I.e. Titanium is in the 4th period and has 4 shells.
Periods are in columns. There are 18 periods, with each group notating the number of valence electrons. I.e. Potassium is in the first group and has one valence electrons. Note* the first 3 rows columns are numbered 1-8.
The periodic table is divided into groups as shownmore groups.
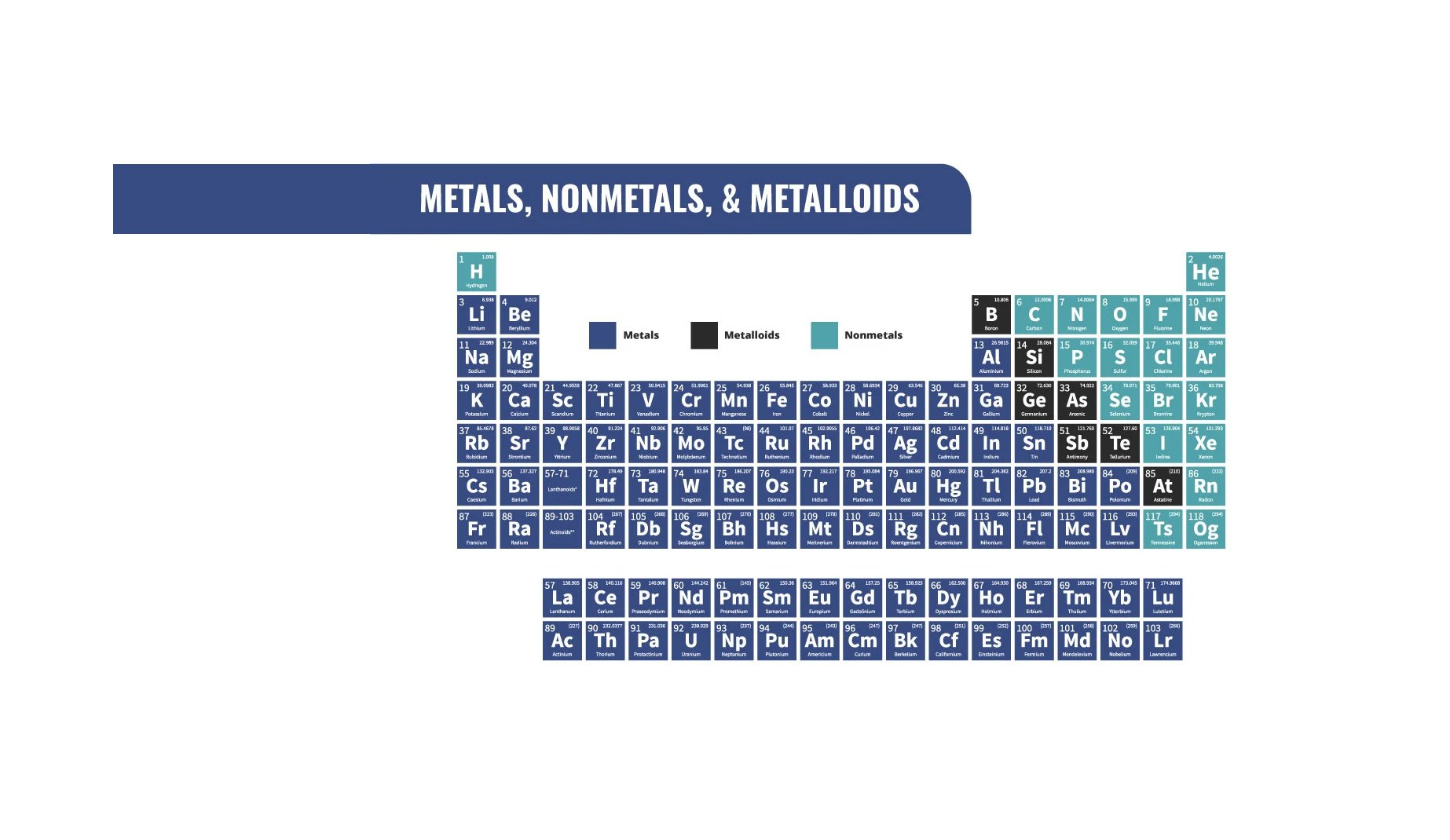
In the periodic table the groups are labeled to make different groups based on properties.

metals vs non-metals
Ions
Ions- a positively or negatively charged atom or group of atoms
Monoatomic Ions- an element that is charged
Polyatomic Ions- a compound that is charged
Cations are positively charged ion (lost electrons)
Anions are negatively charged ion (gains electrons)
The reason an element/compound will eject of gain electrons to satisfy electric stability
This is called the Octet rule (except Hydrogen and Helium), where it is said that most atoms should have 8 electrons in their valence shell.
Hydrogen and Helium use the duet rule, where they need 2 in the outer shell.
When doing a visual representation, use square bracket and put the charge in the upper right corner (like exponents)
When the charge of an ion is one you can omit the one and keep the sign.
ALL METALS are Cations
Non-metals are Anions
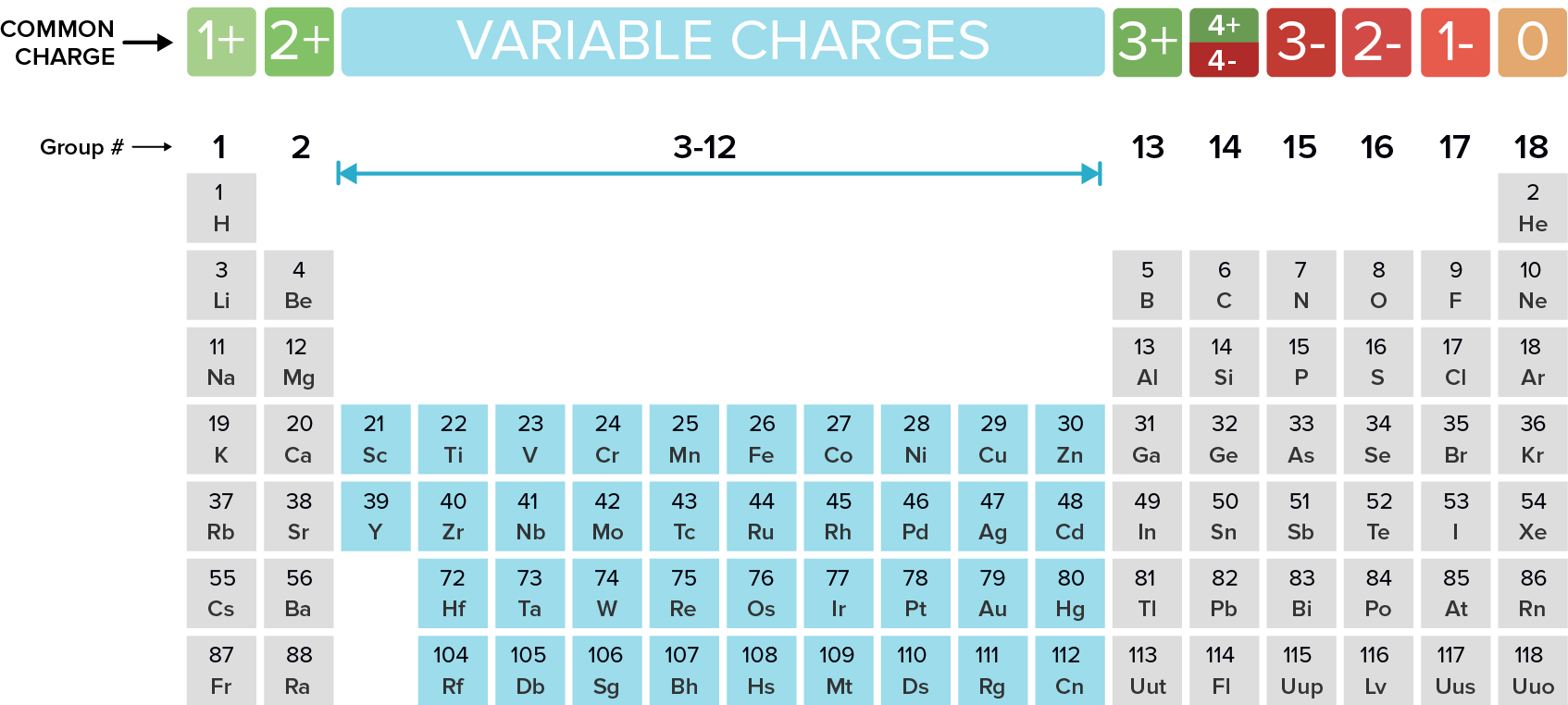
Any cation (metal) will be named the metal’s name followed by ‘cation’.
An anion (Non-metal) will be named the elemental name + ide. Like fluorine becomes Flouride
Electron Transfer and Ionic Bonding
All metals are always Cationic and Non-metals are Anionic
Compound are all types of bonds
All molecules must have a Covalent Bond
An ionic compound must bond a Metal (cationic) and non metal (anionic)
The electrons transfer from the Metal to the Non-metal
Once this occurs the oppositely charged ion join together in to a lattice.
Oppositely charged ions attract each other to form an electrostatic attraction or an Ionic bond.
Model
Example
does not show
chemical formula
NaCl
Charges
Lattice structure
Dot and cross diagram
Lattice structure
Ionic bonds
2D diagram
How ions were formed
More than one layer
3D diagrams
Charges
That there are no spaces between ions
Ionic bonds are quite strong but brittle.
When an ionic compound is exposed to a external force, the layers will slide and like charges will be adjacent. The like charges repel, causing propulsion of bonded section of a lattice leading to smaller pieces.
When bonding polyatomic atoms, you should put brackets around the polyatomic compound before adding the subscript. Ie. Cu2(PO4)2
Electronic Transfer Diagrams
Draw an electron shell diagram of the neutral metal and non-metal
Add a ‘+‘ between them
Draw an arrow leading from each valence in the metal to the valence shell of the non-metal
Add an arrow towards the resulting ions
Draw an electron shell diagram of the resulting cation/s and anion/s
Write the chemical symbol of the metal and the non-metal in the centre of the electron shell diagram, taking care of the electron shell diagram, taking care to add charges to your ions.
Chemical Formulae
The name of the cation (metal) remains the same but without the ending cation and the anion (non-metals) get -ide ended to them.
Worked Example: Lithium Oxide
Write the symbol and charge of the two ions forming the ionic compound: Li^{+} and O^{+2}
Use the shortcut method to find the number of atoms each in the compound (all ionic compounds should be neutral): Therefore we need 2 lithium atoms for one oxygen atom.
Determine the formula of the ionic compound. Write the symbol of cation first. The number 1 should not be written as a subscript: Li2O
Simplify the formula: Ca2O2 is not right. CaO is right
Naming Ionic compounds
Transition metals can take on more than one charge, in this case indicate the charge using roman numerals which are in brackets.
Start with the positive ion or cation (the metal)- ALWAYS- and then follow it with the negative ion or anion (the nonmetal).
Drop the word cation after the positive ion
Metallic bonds
IV: the variable changed by the experimenter, therefore the thing the experiment is exploring
DV: the variable which changes due to the IV, therefore the thing that the experimenter measures.
Control Variable: the variable which is kept constant to ensure the results are accurate.
Metallic bounds are made of metal (which means the structure only has Cations)
can only be made of cations
metallic bonding occurs in pure metals
metals occur as crystal lattices
this is because metallic atoms tend to lose their outer shell electrons easily
this turns the metals atoms in positively-charged cations
the cations form a metallic lattice structure, in which electrons from each metal atom overlap with each other, forming a sea of electrons that can flow between all the metal ions
Metallic bonds are very malleable as their structure allows for sliding. However, they are hard to break apart.
Alloys are generally stronger than normal metallic bonds because in a pure element can slide around (as it is very organized), whereas in alloys the larger atom will break the structure, making sliding of atoms harder.
Ionic Properties
The purpose of an atom is to achieve stability (8 valance electrons)
To melt ionic compounds you need a lot of energy because the electrostatic bond is very strong.
Ionic compounds dissolve in water because the kinetic energy of the water breaks down the electrostatic bond
Electronegativity refers to the ability of an element to attract electrons to itself.
Group 7/17 is the most electronegative because it is desperate to get the last electron it needs to achieve stability.
In a covalent compound there is no anions or cations instead the only have a partial charge.
Polarity is the separation of electric charge within a molecule, creating a difference in charge distribution
Polarity is about the elements that make up the compounds and there electronegativity and geometry
Because of the partial negative charge of oxygen, the cations will be swarmed by oxygen atoms and the hydrogen atoms will coat the anions. Calcium Carbonate is an exception because Calcium Carbonate is extremely strong.
Voltage is another word for electrical energy.
Dissolved ionic compounds are charged because the atoms are cations and anions.
Cations and anions are free moving charged molecules.\
The movement of charged particles is called a current.
Currents have direction. And the cations and anions move in different direction
Molten ionic compounds are also conductive.
Covalent Bonding
Occurs between non-metals (ONLY)
In covalent bonding, atoms share electrons to achieve a full outer shell, resulting in the formation of molecules.
They must share electrons in pairs that have opposite spins, which allows them to create stable bonds and not repel.
The electrons pair is called a bonding pairs
A compound made with the same element is called a di-atomic compound.
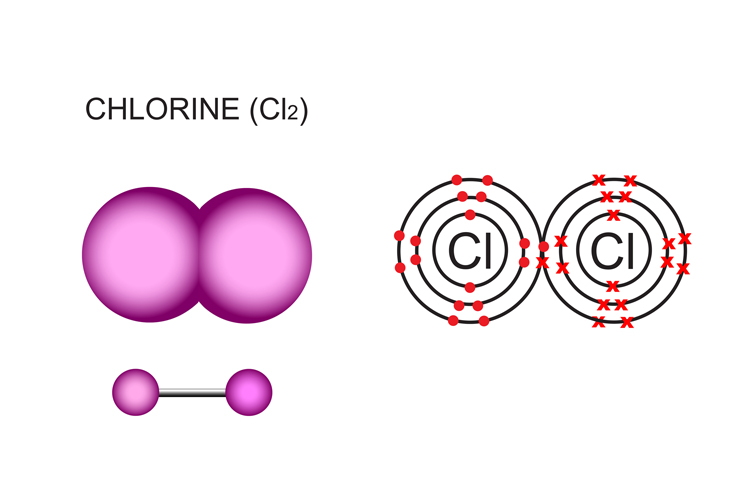
The atoms are equal in strenght (they have the same level of electronegativity)
All halogens can bond with themselves.
1 bounding pair is a SINGLE CHEMICAL BOND.
A lewis diagram only represents the valence shell and portrays the electrons in pairs.
A line diagram only represents the number of bonding pairs.
Electronegativity