pKa values
0.0(0)
Card Sorting
1/16
There's no tags or description
Looks like no tags are added yet.
Last updated 3:53 PM on 1/14/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
1
New cards
Hydroiodic acid (HI)
pKa ≈ −10 (very strong acid)
2
New cards
Hydrobromic acid (HBr)
pKa ≈ −9 (very strong acid)
3
New cards
Hydrochloric acid (HCl)
pKa ≈ −8 (very strong acid)
4
New cards
Sulfuric acid (H₂SO₄)
pKa ≈ −3 (strong acid)
5
New cards
Hydronium ion (H₃O⁺)
pKa ≈ −1.7
6
New cards
Hydrofluoric acid (HF)
pKa ≈ 3.2 (weak acid despite HF bond)
7
New cards
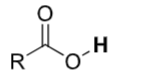
Carboxylic acids
pKa ≈ 4–5
8
New cards
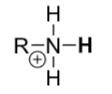
Protonated amines
pKa ≈ 9–11
9
New cards
Water (H₂O)
pKa ≈ 15.7
10
New cards
Alcohols
pKa ≈ 16–18
11
New cards
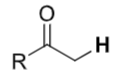
Ketones
(α-hydrogens) pKa ≈ 20–24
12
New cards
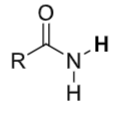
Amides
pKa ≈ 25
13
New cards
Terminal alkynes
pKa ≈ 25
14
New cards
Hydrogen (H₂)
pKa ≈ 35
15
New cards
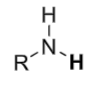
Amines
pKa ≈ 35
16
New cards
Alkenes
pKa ≈ 44
17
New cards
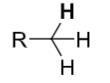
Alkanes
pKa ≈ 50