AP Chem Unit #4
1: Water, the Common solvent
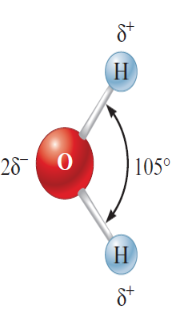
Water
POLAR molecule
unequal distribution of charge
polarity facilitates water’s ability to dissolve compounds
Ability to DISSOLVE many substances
homogenous mixture, soltuion, aqueous
Shape
collection of H2O molecules
H-O-H 105 degree
O-H covalent in nature
unequal sharing between the atoms
oxygen has a greater attraction for electrons → helps it gain slight excess of negative charge
Nacl(s) → Na(s) + Cl2(g) : CHEMICAL
Nacl(s) → Na+2(aq) + Cl-1 (aq) : PHYSICAL DISSOLUTION
δ - delta (partical charge symbol
Hydration
process by which positive ends of H2O molecules are attached to negatively charged ions and vice versa
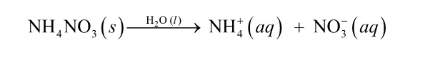
Solubility
solubility of ionic substances in water varies depending on
the attraction among ions
the attraction of ions for water molecules
polar and ionic subtances are more soluble in water than nonpolar substances (like likes like) -
ionic conducts electricity
nonionic substances are soluble because they are polar - cannot conduct electricity
2: Electrical conductivity
ability of a solution to conduct electric currents
solute: being dissolved
solvent: dissolving medium
nonpolar covalent = insoluble
ionic compounds = soluble
electrolyte
solute that dissolves in water to produce a solution that can conduct electricity
strong electrolytes
highly efficient conductors of current in aqueous solutions = many ions
completely pulled apart in water
NaCl - salts
soluble salts (ionic compounds)
strong acids
strong bases
weak electrolytes
conduct small current in aqueous solutions = few ions
partially dissolved
acetic acid - weak acid
nonelectrolytes
do not conduct current in aqueous solutions= no ions
sugar! alcohol and molecules
Svante Arrhenius
definition of acid and bases
indentified the basis for the conductivity properties of solutions
extenet to which a solution can conduct an electric current directly depends on the number of ions present
Soluble salts
contrian array of cations and anions
disintegrate and undergo hydration when the salt dissolves
the attraction of ions for water molecules is greater
Nature of acids
when dissolved in water, acids act strong electrolytes - produce ions
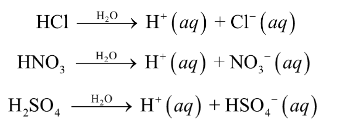
Acids: substance that produces H+1 ions when it is dissolved in water
polarity of water helps produce H+1 ions
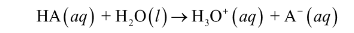
strong acids
every molecule fully dissociates (completely ionized)
ex: HCl, HNO3 H2SO4
arrow to the right
produces max moles of ions possible
Strong bases
soluble ionic compounds that contain the OH ion
fully dissociate when dissolved in water, cations and OH ions separate and move independently
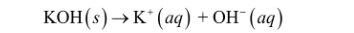
Weak electrolytes
Weak bases: resulting solution will be weak electrolyte ( partical dissociation)
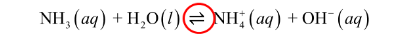
Weak acids: dissociate only a slight extent
double arrow indicates that the reaction can occur in either direction
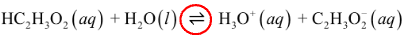
small degree of ionization in water
weak acids and weak bases
formula of acids
atoms that produce H ion is acid
OH is base
Non electrolytes
substances that dissolve in water but do not produce any ions
3: Composition of solutions (molarity M)
commonly used expression for concentration
expressed as moles of solute per volume of solution in liter
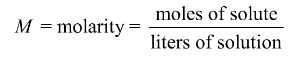
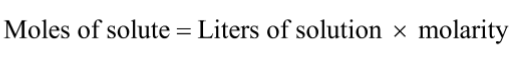
Standard solution
solution whose concentration M is accurately known
process of preparation
1. place a weighed amount of the solute into a volumetric flask and add a small amount of water
2. dissolve the solid swirling the flask
3. add more water until the level of the solution reaches the mark etched on the flask
mix by invention
label with name
Dilution
adding water to a concentrated solution to achieve molarity desired for a particular solution
MOLES NOT CHANGED
pipet
device used for the accurate measurement and transfer of a given volume of solution
4: Types of chemical reaction
Types of solution reactions
precipitation reactions
when two solutions are mixed, a precipitate separates from the solution
precipitate
insoluble solid that is formed in a precipitation reaction
acid base reactions
oxidation-reduction reactions (redox)
6: Equations used to represent reactions in solution
formula equation
overall reaction - stoich
complete ionic equation
all reactants and products (that are strong electrolytes) are represented as ions
net ionic equation
includes only those solution components that undergo change
no spectator ions
spectator ions
ions that do not directly participate in a reaction
7: Problem solving stragety
solving stoich probelms for reactions in a solution

8: Acid-base reactions
definitions
Bronsted-Lowry
acid = proton donor
base = acceptor
Aerenous
OH - base
H - Acid
Neutralization reaction 1:1
general name given to acid-base reactions
acid is “neutralized” when enough base reacts exactly with it in a solution
Strong acid + strong base → ionic compound and water
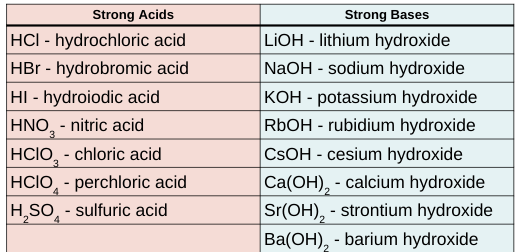
Alkali + Alkaline earth = bases (STRONG)
Problem solving strategy
list the species present
decide what reaction occurs
write balanced net ionic equation
calculate moles of reactant
for reaction in a solution use the volumes of the original solutions and their molarities
determine the limiting reactant, sometimes
calculate the mole of the required reactant product
covert to grams
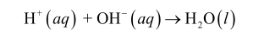
strong

Volumetric analysis
titration!
delivery of a tritrant into analyte
titrant
solution of known concentration (in buret)
analyte
solution containing the substance being analyzed (unknown)
Equivalence (stoichiometric) point
marks the point in titration where enough titrant has been added to react exactly with the analyte
indicator
methyl red that changes the color at the equivalence point
Phenolphtalein
pink in basic
endpoint
point where the indicator actually changes color
NO LIMITING REACTANT
9:Oxidation Reduction
transfer of one or more electrons
everything else that isnt acid-base or precipitant
Oxidation states (identifying oxidation numbers)
the oxidation state of an uncombined elemnt is zero
Na, Ca, H2 and HOFBrINCl - NO redox
the sum of the oxidation states of all the atoms or ions in a neutral compound is zero
Na+1Cl-1
# of electrons lost = # of electrons gained
The more electronegative element = negative oxidation state
less electronegative one is given a positive oxidatioon state
F = most electronegative
O = second most electronegative
SUM of the oxidation states in an ion is = the charge on the ion
IDENTIFYING
some elements almost always have the same oxidation states in their compounds
group 1 = +1
group 2 = +2
Oxygen = -2
Flourine = -1
Hydrogen = +1 (when covalent)
When hyride = -1
Compound order= least electronegative most
Na Cl
termanoligy
oxidation
increase in oxidation state = lose electrons (+ ion)
reduction
decrease in oxidation state = electron gain (- ion)
reducing agent
electron donor (oxidized) = lost electrons
oxidizing agent
electrong acceptor ( reduced) = gain electrons
LEO the lion says GER
Losing Elections Oxidation
Gain Electrons Reduction
10: Balancing Oxidation-Reduction
2 methods
inspection
always try
is the reaction redox?
assign and compare oxidation #’s
determine net charge in oxidation # for each
determine a ratio of oxidized to reduced atoms that would = a net increase in oxidation number equal to net decrease