Particle Theory
0.0(0)
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Last updated 3:24 PM on 10/21/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
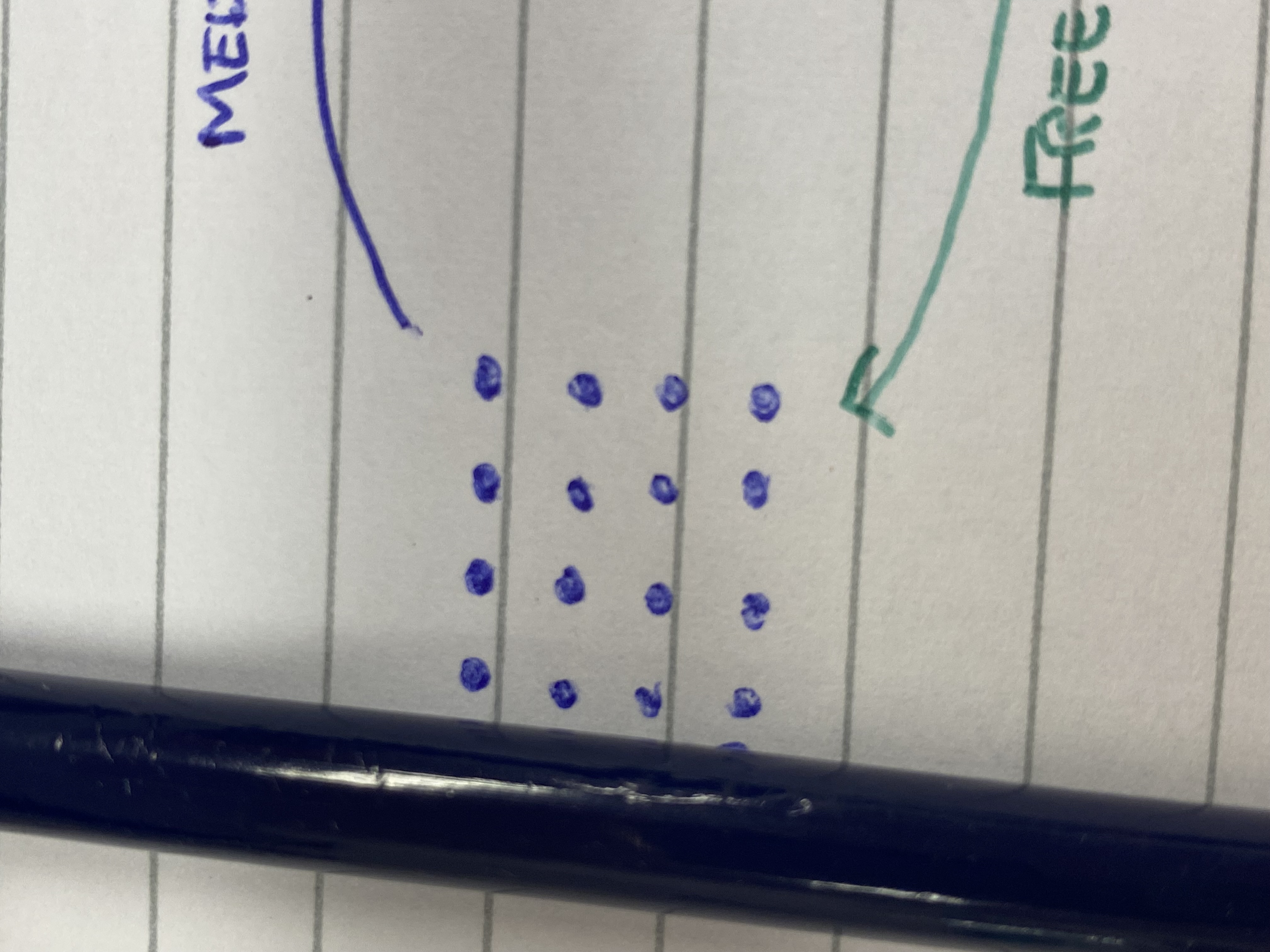
Solid particle
2
New cards

Liquid particles
3
New cards
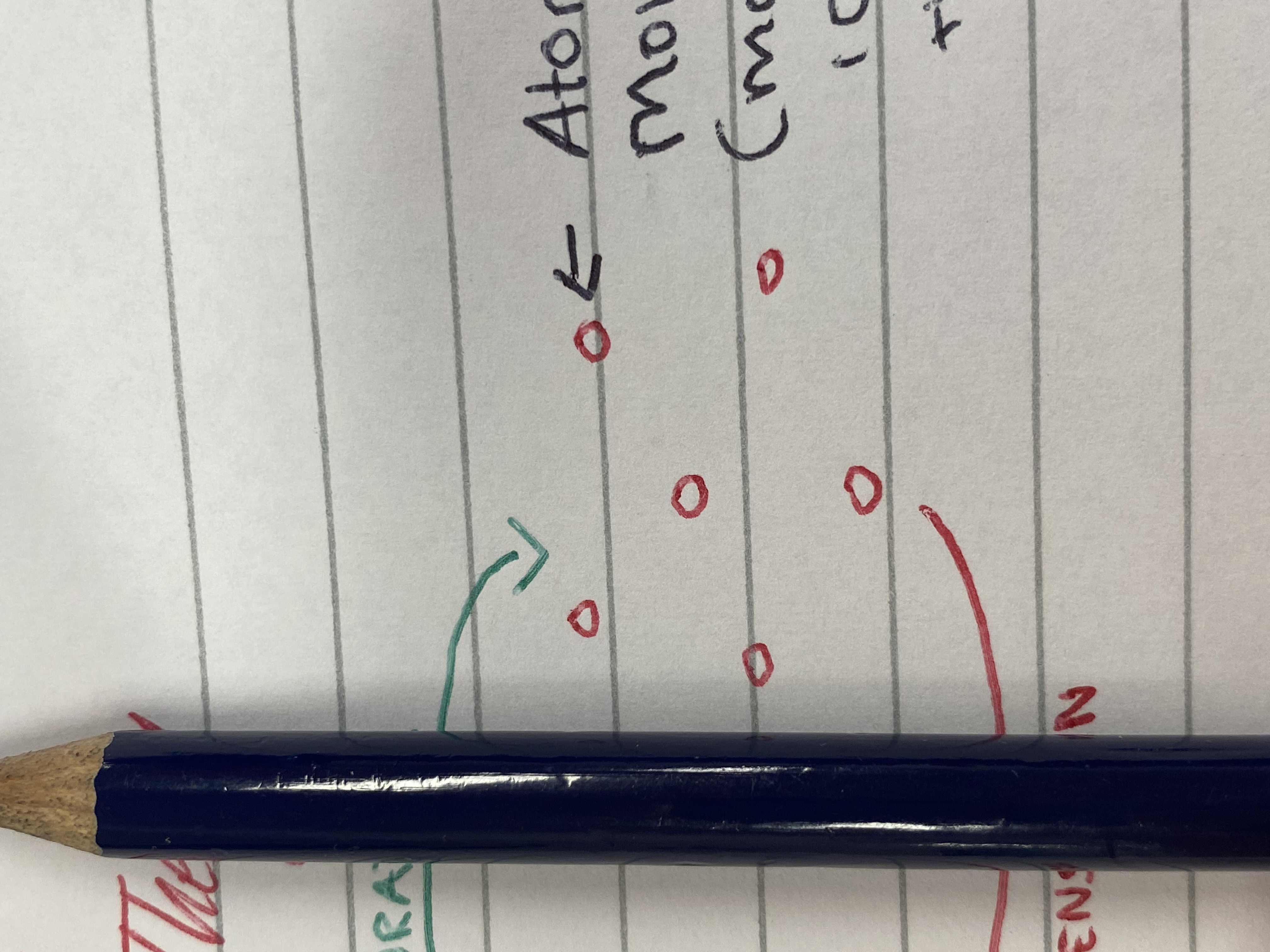
Gas particles
4
New cards
Diffusion
Chemicals tend to move from areas of high concentration to areas of low concentration
5
New cards
Brownian Motion
The random movement of particles in a gas
6
New cards
Ideal gas
Imaginary gas whose properties fit perfectly with the assumptions of kinetic molecular theory
7
New cards
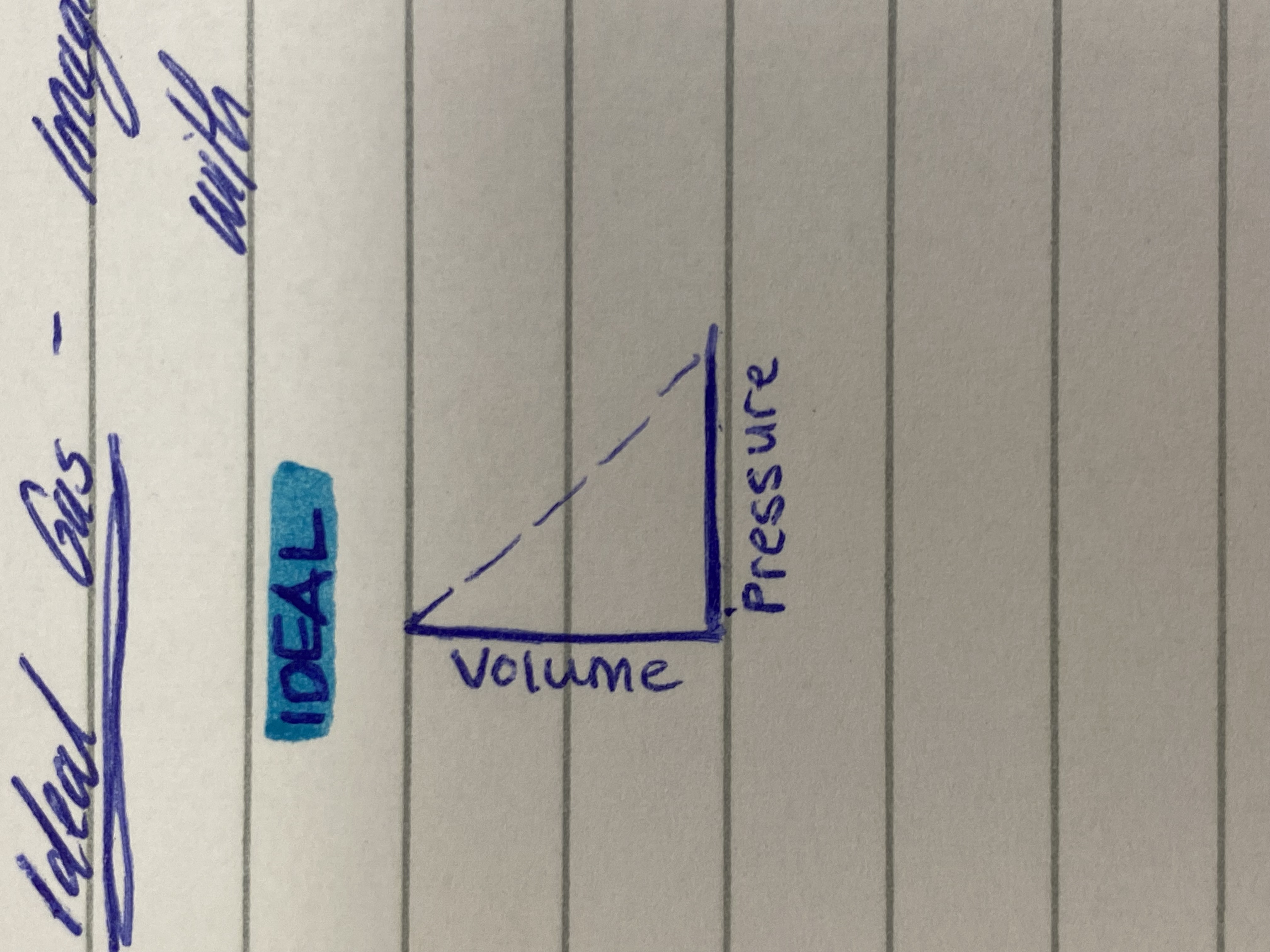
Ideal gas graph
8
New cards
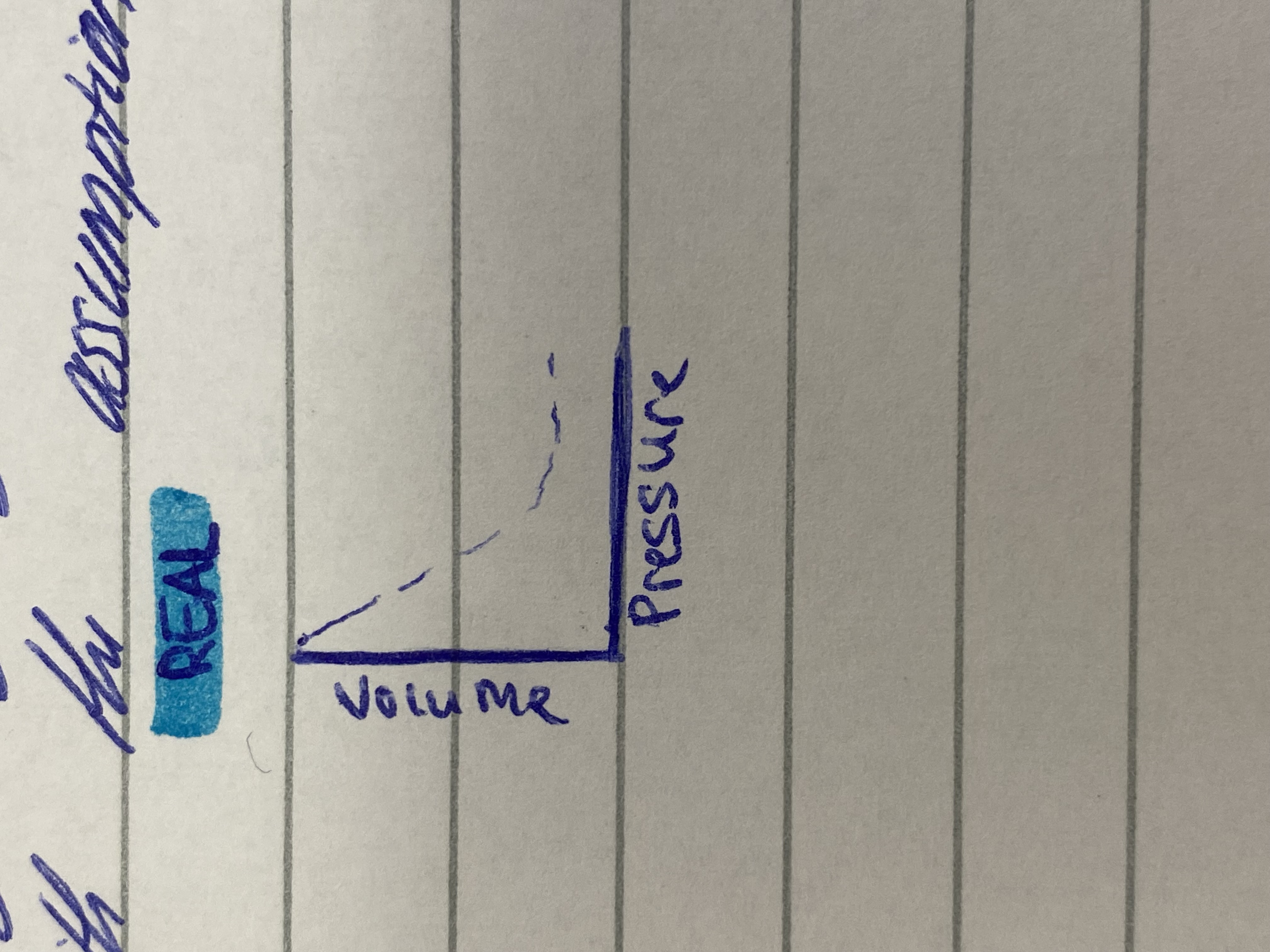
Real gas graph