8.2.3 Gene expression and cancer
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
What is the initial cause of tumour formation?
Mutations in DNA or genes controlling mitosis can lead to uncontrolled cell division
What defines a mass of cells as a tumour?
Tumour formed if mutation results in mass of abnormal cells
Malignant tumour = cancerous, can spread by metastasis
Benign tumour = non-cancerous
Describe the growth rate of benign tumours.
Usually grow slowly as cells divide less often
Describe the cell differentiation in benign tumours.
Cells are well differentiated / specialised
Describe the nuclei in benign tumour cells.
Cells have normal, regular nuclei
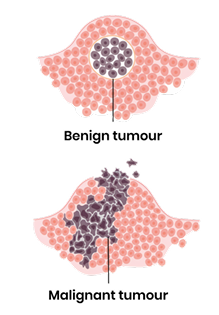
Describe the borders and invasiveness of benign tumours.
Well defined borders and often surrounded by a capsule so do not invade surrounding tissue
Do benign tumours spread (metastasize)? Why or why not?
Do not spread by metastasis as cell adhesion molecules stick cells together
How are benign tumours typically treated, and what is the prognosis?
Can normally be removed by surgery and they rarely return
Describe the growth rate of malignant tumours.
Usually grow faster as cells divide more often
Describe the cell differentiation in malignant tumours.
Cells become poorly differentiated / unspecialised
Describe the nuclei in malignant tumour cells.
Cells have irregular, larger and darker nuclei
Describe the borders and invasiveness of malignant tumours.
Poorly defined borders and not encapsulated so can invade surrounding tissues (growing projections)
Do malignant tumours spread (metastasize)? Why or why not?
Spread by metastasis - cells break off and spread to other parts of the body, forming secondary tumours due to lack of adhesion molecules
How are malignant tumours typically treated, and what is the prognosis?
Can normally be removed by surgery combined with radiotherapy or chemotherapy but they often return
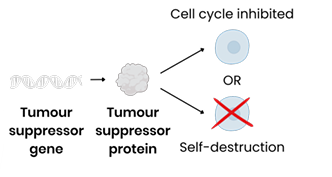
What is the function of tumour suppressor genes?
Code for proteins that:
Inhibit or slow cell cycle if DNA damage detected
OR cause self-destruction (apoptosis) of potential tumour cells if damaged DNA can’t be repaired
How does a gene mutation lead to a non-functional tumour suppressor protein?
Changes sequence of base triplets in DNA so changes sequence of codons on mRNA
So changes sequence of amino acids in the encoded polypeptide
So changes position of hydrogen / ionic / disulphide bonds (between amino acids)
So changes tertiary structure (shape) of protein which may result in a non-functional protein.
How do epigenetic changes prevent the production of a tumour suppressor protein?
Decreased histone acetylation OR increased DNA methylation → promoter region less accessible to transcription factors
So prevents binding of RNA polymerase to promoter region, inhibiting transcription
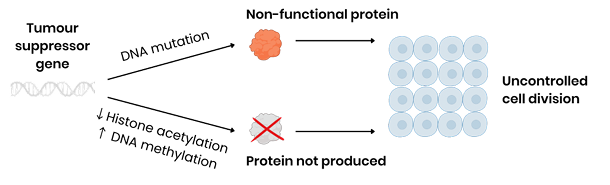
What is the ultimate consequence of a lost or non-functional tumour suppressor gene?
Both lead to uncontrolled cell division (cell division cannot be slowed)
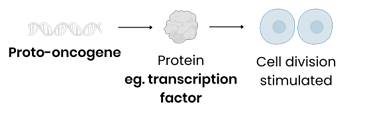
What is the function of proto-oncogenes?
Code for proteins that stimulate cell division (eg. through involvement in signalling pathways that control cell responses to growth factors)
What is an oncogene?
An oncogene is a mutated / abnormally expressed form of the corresponding proto-oncogene.
How does a mutation cause an oncogene to produce a permanently active protein?
Mutation in DNA base sequence → overproduction of protein OR permanently activated protein
By leading to change in amino acid sequence which changes protein tertiary structure
How do epigenetic changes cause an oncogene to overproduce protein?
Decreased DNA methylation OR increased histone acetylation → promoter region more accessible to transcription factors
By stimulating binding of RNA polymerase to promoter region, stimulating transcription
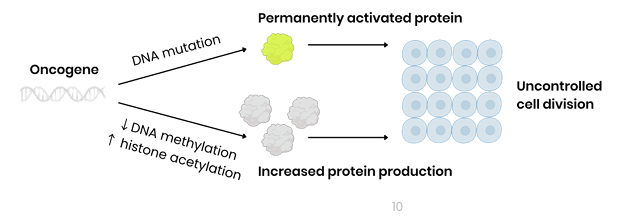
What is the ultimate consequence of an activated oncogene?
Both lead to uncontrolled cell division as cell division is permanently stimulated
Why do tumours require mutations in both alleles of a tumour suppressor gene?
One functional allele of a tumour suppressor gene can produce enough protein to slow the cell cycle OR cause self-destruction of potential tumour cells → cell division is controlled
Why do tumours require mutations in only one allele of an oncogene?
One mutated oncogene allele can produce enough protein to lead to rapid / uncontrolled cell division
What is the general relevance of epigenetics in cancer treatment?
Drugs could reverse epigenetic changes that caused cancer, preventing uncontrolled cell division
How could a drug target an oncogene?
Increasing DNA methylation OR decreasing histone acetylation of oncogene
By inhibiting binding of RNA polymerase to promoter region, inhibiting transcription / expression
How could a drug target a tumour suppressor gene?
Decreasing DNA methylation OR increasing histone acetylation of tumour suppressor gene
By stimulating binding of RNA polymerase to promoter region, stimulating transcription / expression
What is the first step linking high oestrogen to some breast cancers?
Some breast cancers cells have oestrogen receptors, which are inactive transcription factors
What happens when increased oestrogen binds to these receptors?
2. If oestrogen concentration is increased, more oestrogen binds to oestrogen receptors, forming more oestrogen-receptor complexes which are active transcription factors
Where do these active complexes go, and what genes do they affect?
3. These complexes bind to promoter regions of genes that code for proteins stimulating cell division
What is the final result of this process?
4. This increases transcription and expression of these genes, increasing the rate of cell division
How do drugs that mimic oestrogen structure help treat breast cancer?
Drugs bind to oestrogen receptors (inactive transcription factors), preventing binding of oestrogen
So no / fewer transcription factors bind to promoter regions of genes that stimulate the cell cycle