Chem 1310 Problems
1/13
Earn XP
Description and Tags
How to Solve
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
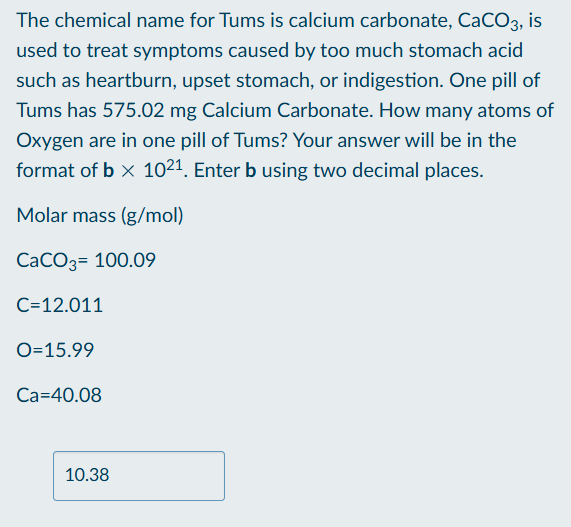
How to find amount of atoms in a molecule
Find the percentage of the element present in one mol of molecule
Multiply by the mass of the molecule given
Convert mass of element to mol
Multiply mol by the subscript of the molecule
Convert mol to atoms by multiplying by 6.022 × 1023

How to find unknown percent abundance
Subtract percentage of known isotopes to find percent of unknown isotopes (n)
Set given isotope abundance to x
Multiply each isotope by percent abundance, including (n -x) and x
Set equation equal to average atomic mass and solve for x
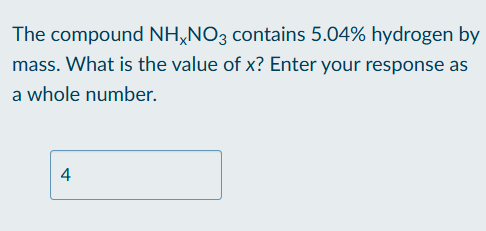
Calculate number of atoms of element in molecule based on molecule composition
Calculate molar mass of compound based on given information
Set up percentage equation based on x
Solve for x

Find mass required for percentage yield
Utilize given actual value to find theoretical value
Convert theoretical value to mol
Find mol necessary of reactant to create that much product
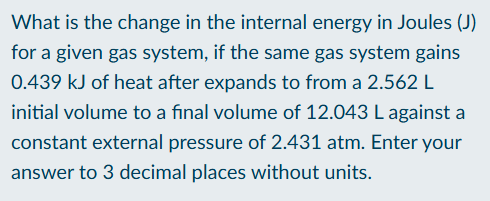
Find change in internal energy for a given system based on pressure
Use the law of thermodynamics to find change in energy = q + w, where work = -pressure x change in volume
Calculate change in volume for the system
Plug in given pressure and change in volume to find work
Plug given values to find change in energy

Find change in heat for a coffee cup calorimeter
mc c (Thot - Tfinal) = mcol c (Tfinal - Tcold) + ccal (Tf - Tcold)

Determine heat of reaction
Cancel out compounds necessary to get final reaction
When reversing reactions, multiple deltaH by -1
When multiplying reactions by c, multiplye deltaH by c
Add the new deltaH for each reaction to determine overeall heat of reaction

How to find mol of a compound for a gas reaction
Use ideal gas law to find mol of gas
Use stoichiometric relationship to find mol of solid
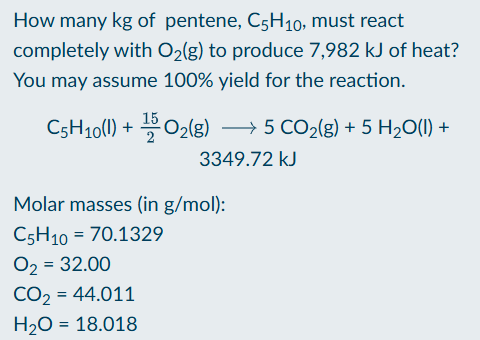
How to find heat of reaction
Divide given deltaH by deltaH of reaction
Find stoichiometric ratio between mol of reaction and mol of element A
Multiply mol of A by molar mass of A to find necessary A

How to find partial pressure
Find total number of moles
Utilize the relationship pressure ratio = mol ratio to find partial pressure for given substance
Subtract Pi from Ptotal to find P of the other compound
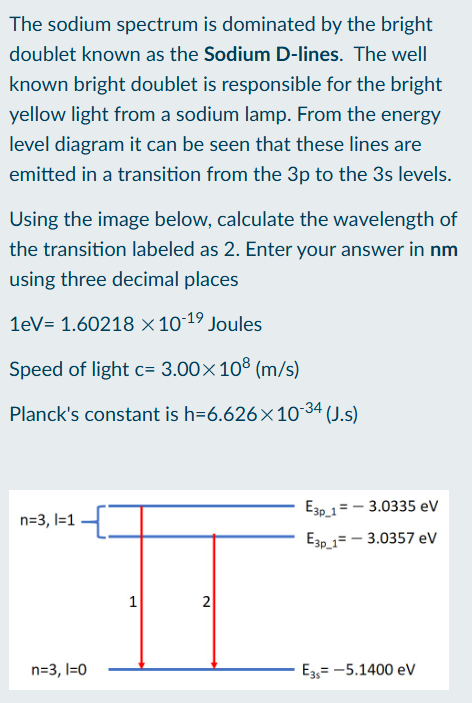
Calculate wavelength of transition state
Find the difference between each transition state
Convert eV to Joules
Use energy-wavelength relation to find wavelength of transition

Calculate moles of photon in laser pulse
Calculate Ephoton
Find number of photons in laser beam by dividing Ebeam by Ephoton
Divide number of photons by Avogadro’s number
Calculate time for decomposition
Find fraction of compound decomposed
Plug in given mass into integrated rate law
Find time necessary for decomposition

Solve for constant of equilibrium for pressure
Find the molarity of gas
Create an ice table to find x based on the starting numbers
Convert found equilibrium values to partial pressures
Solve for Kp
Find the partial pressures based on the value of x
Add partial pressures together