Polyatomic Ions
0.0(0)
Card Sorting
1/20
Earn XP
Description and Tags
Last updated 6:42 PM on 5/29/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
1
New cards
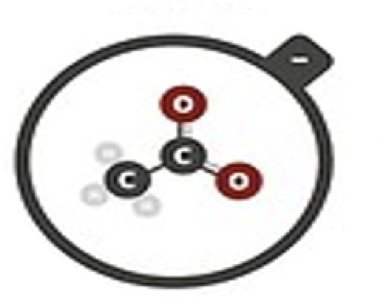
Acetate
C2H3O2–
2
New cards
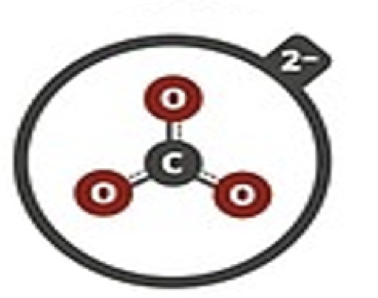
Carbonate
CO32–
3
New cards
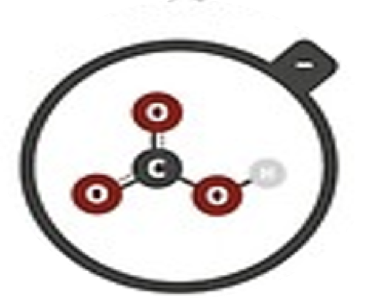
Hydrogen carbonate
HCO3–
4
New cards
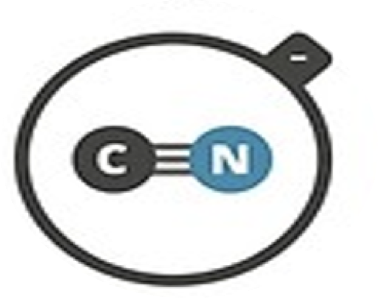
Cyanide
CN–
5
New cards
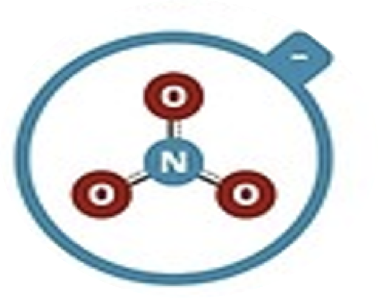
Nitrate
NO3–
6
New cards
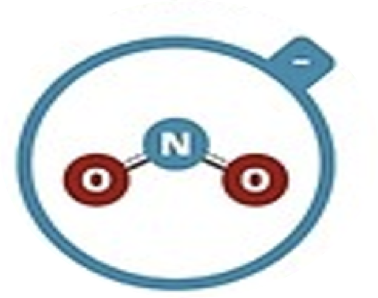
Nitrite
NO2–
7
New cards
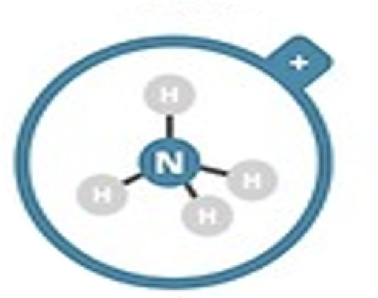
Ammonium
NH4+
8
New cards
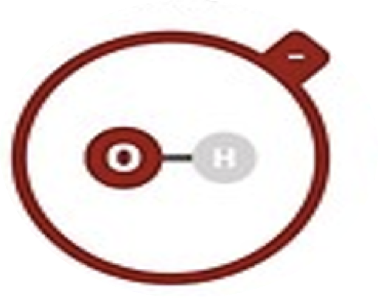
Hydroxide
OH–
9
New cards
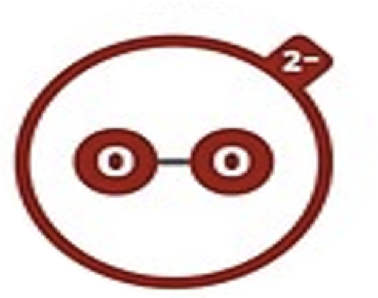
Peroxide
O22–
10
New cards
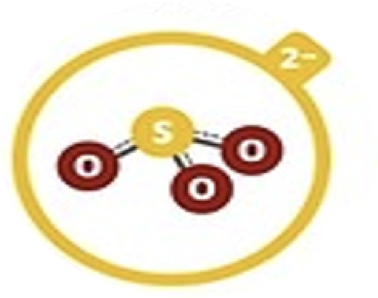
Sulfite
SO32–
11
New cards

Sulfate
SO42-
12
New cards
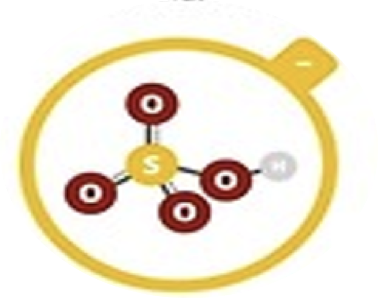
Hydrogen sulfate
HSO4–
13
New cards
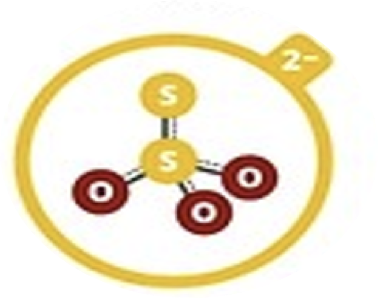
Thiosulfate
S2O32–
14
New cards
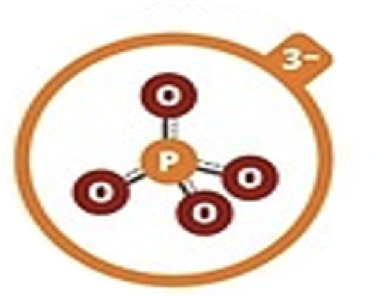
Phosphate
PO43–
15
New cards
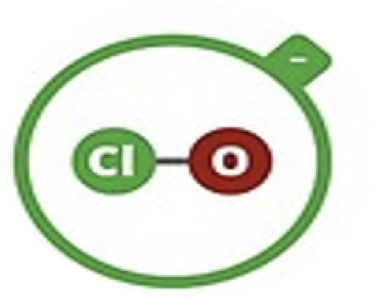
Hypochlorite
ClO–
16
New cards
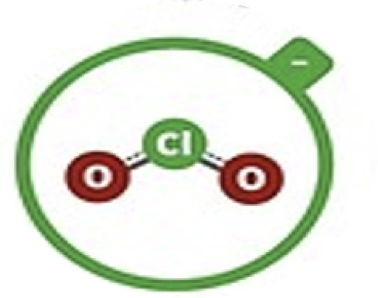
Chlorite
ClO2–
17
New cards
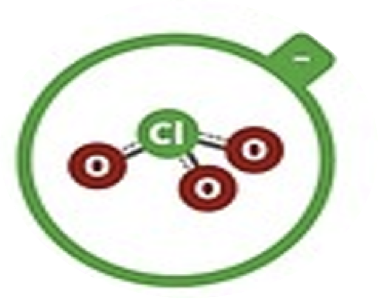
Chlorate
ClO3–
18
New cards
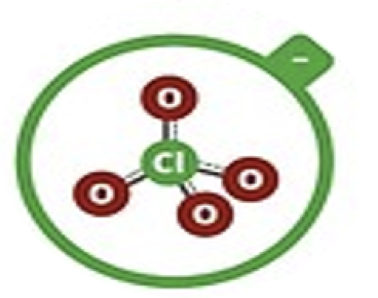
Perchlorate
ClO4–
19
New cards
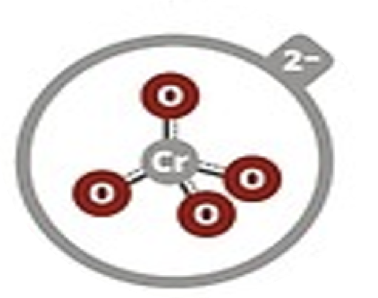
Chromate
CrO42–
20
New cards
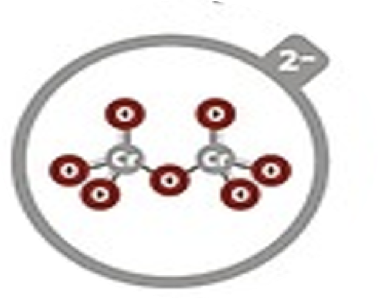
Dichromate
Cr2O72–
21
New cards
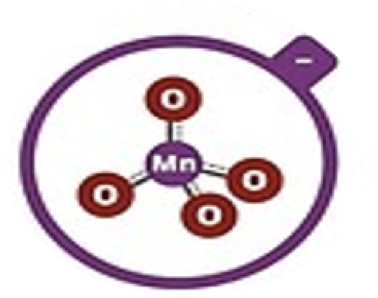
Permanganate
MnO4–
Explore top notes
Explore top flashcards
CRISC - Certified in Risk and Information Systems Control term definition - Part 53
20Updated 1207d ago0.0(0)
CRISC - Certified in Risk and Information Systems Control term definition - Part 53
20Updated 1207d ago0.0(0)