Acyl-CoA synthesis and transport + BETA OXIDATION + UNSATURATED and ODD-NUMBER FA + PEROXISOMES (slide deck 2)
1/29
Earn XP
Description and Tags
steps and structures
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
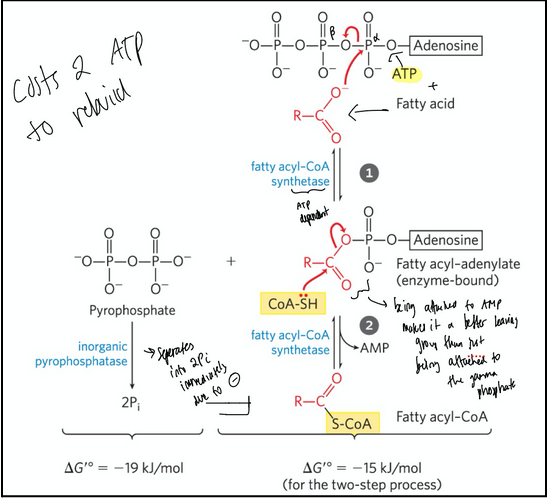
formation of a fatty acyl-CoA (activation of fatty acids before beta oxidation)
Fatty acyl-CoA = contains a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A
High energy compound
Formation is made more favourable by the hydrolysis of two ATP bonds
Steps:
A fatty acid reacts with ATP. Instead of going ATP—> ADP like in most reactions, it uses ATP —> AMP + PPi (pyrophosphate). This creates an acyl-adenylate intermediate (fatty acid carboxyl group attached to AMP’s phosphate).Enzyme = fatty acyl-CoA synthetase
Coenzyme A (CoA-SH) attacks the acyl-adenylate. This displaces AMP, forming fatty acyl-CoA. Enzyme = fatty acyl-CoA synthetase
This reaction is strongly favorable because pyrophosphate (PPi) released in step 1 is rapidly hydrolyzed to 2 Pi by pyrophosphatase. This makes the reaction essentially irreversible.
Costs 2 ATP because regenerating ATP from AMP requires 2 phosphorylations
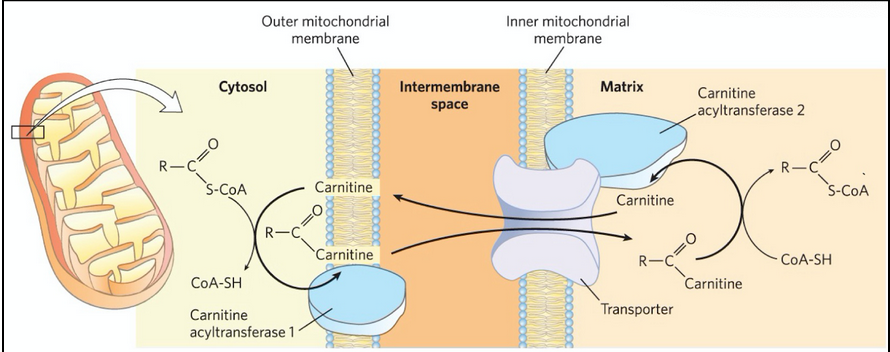
Carnitine
compound that transports fatty acyl-CoAs destined for mitochondrial oxidation across the inner mitochondrial membrane
Carnitine acyl-transferase 1, CAT 1
(carnitine palmitoyltransferase 1, CPT1)
catalyzes a transesterification reaction to transiently (temporarly) attach a fatty acyl-CoA to the hydroxyl group of carnitine to form fatty acyl-carnitine.
located on outer mitochondrial membrane
Acyl-carnitine/carnitine cotransporter
allows the passive transport of the fatty acyl-carnitine ester
Moves one carnitine into the intermembrane space as one fatty acyl-carnitine moves into the matrix
the two pools of coenzyme A in the carnitine shuttle
One pool is in the cytosol and the other is in the mitochondria
Cytoplasmic CoA and fatty acids are streamed towards lipid biosynthesis
Matrix CoA and fatty acids are streamed towards lipid breakdown (oxidative degradation of pyruvate, fatty acids, and some amino acids)
carnitine acyltransferase 2 (CAT2)
CPT2
transfers the fatty acyl group from carnitine back to coenzyme A to regenerate fatty acyl-CoA and free carnitine
Located on the inner face of the inner mitochondrial membrane
the carnitine shuttle is a major control point… (regulation)
Carnitine-mediated entry is the rate-limiting step for oxidation of fatty acids in the mitochondria
Carnitine acyltransferase 1 (CAT1) is inhibited by malonyl-CoA, the first intermediate in fatty acid synthesis
Prevents the simultaneous synthesis and degradation of fatty acids
transport of acyl-CoA into the mitochondria
S-CoA group is exchanged for carnitine generating an acylcarnitine by CAT1 on the outer mitochondrial membrane. Fatty-acyl-carnitine then can enter the inner matrix via the transport membrane channel
Translocation into the mitochondrial matrix and exchange of carnitine for CoA by CAT2
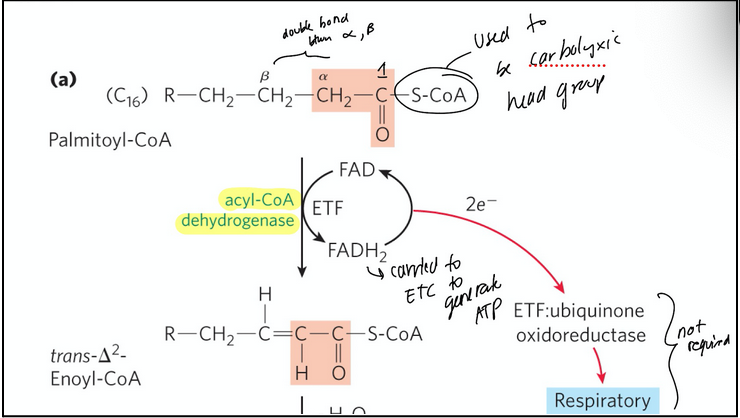
Beta-oxidation step 1
Oxidation reaction forms a double bond between the alpha and beta carbons
acyl-CoA dehydrogenase = flavoprotein with tightly bound FAD (co-factor) that catalyzes the dehydrogenation of fatty acyl-CoA to yield a trans-∆2-enoyl-CoA
One of the three different enzymes (isozymes) performs this reaction depending on the length of the carbon chain of the fatty acid
FAD during the reaction is reduced, while the substrate is oxidized; this produces electrons
FAD → FADH2 and the electrons generated will be used in the ETC to produce ATP
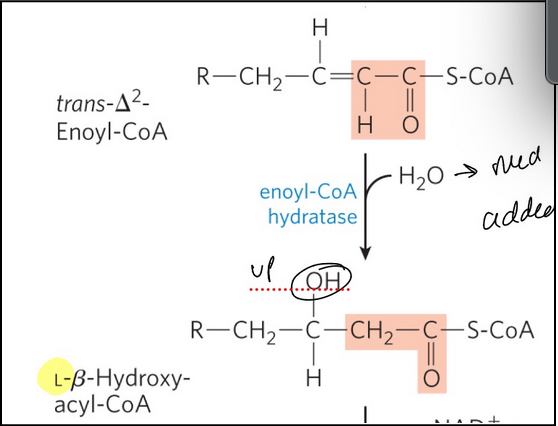
Beta-oxidation step 2
Hydration reactions adds water to the double bond forming L-beta-hydroxy-acyl-CoA
enoyl-CoA hydratase = catalyzes the addition of water to the double bond of the trans-∆2-enoyl-CoA to form L-𝛽-hydroxyacyl-CoA (3-hydroxyacyl-CoA)
Catalyzed in the trifunctional protein
Formally analogous to the fumarase reaction in the citric acid cycle
Hydroxyl added to beta carbon
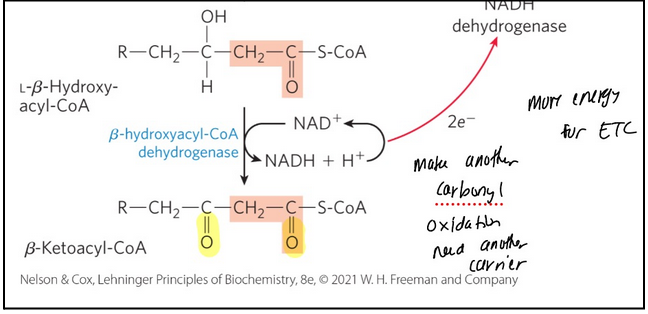
Beta-oxidation step 3
Oxidation reaction forms a keto from the hydroxyl group -- generating beta-ketoacyl-CoA
𝛽-hydroxyacyl-CoA dehydrogenase = catalyses the dehydrogenation of L-𝛽-hydroxyacyl-CoA to form 𝛽-ketoacyl-CoA
Carbonyl group on the beta carbon —> now have a good L.G.
Catalyzed in the trifunctional protein
Enzyme is specific for the L stereoisomer
Closely analogous to the malate dehydrogenase reaction of krebbs
Uses NAD+ → NADH + H+ and 2 electrons go to ETC
NADH dehydrogenase (complex I) = electron carrier of respiratory chain
Accepts electrons from NADH formed in the 𝛽-hydroxyacyl-CoA dehydrogenase reaction
Makes 2.5 ATP whereas FAD makes 1.5
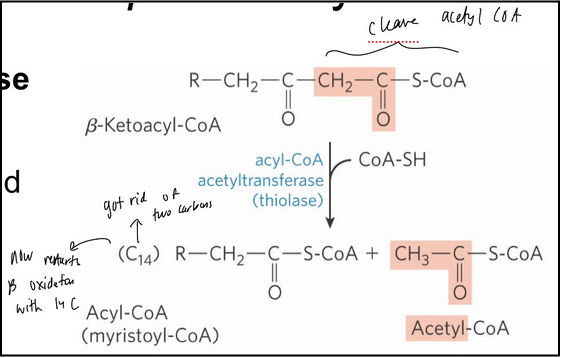
Beta-oxidation step 4
Thiolysis now cleaves the acetyl-CoA (a good leaving group) from the remaining hydrocarbon chain forming a new Acyl-CoA substrate for another round of beta oxidation
acyl-CoA acetyl-transferase (thiolase) = catalyzes the reaction of 𝛽-ketoacyl-CoA with free coenzyme A to yield acetyl CoA and a fatty acyl-CoA shortened by 2 carbons
Catalyzed in the trifunctional protein
isozymes of acyl-CoA dehydrogenase
Similar over all fold
Homologous function and mechanism
Specificity for specific fatty acyl-CoA chains
All bind tightly to the FAD cofactor
Everytime you go through beta oxidation you lose 2 carbons so you have a shorter chain, different isozymes allow for the specificity in binding of different length chains
trifunctional protein (TFP)
Trifunctional protein (TFP) is a multienzyme complex associated with the inner mitochondrial membrane that catlyzes steps 2-4 of the 𝛽-oxidation pathway for fatty acyl chains of 12+ carbons
When the chain is short enough you can overcome hydrophobic issues
The three steps are catalyzed in TFP so the substrates can avoid exposure to the aqueous environment and potential degradation of intermediates by substrate channeling → the enzymes and the active sites are all within the protein nearby so that the products of each step cannot just float away and risk being degraded
It allows for efficient substrate channeling
TFP is a heterooctamer of 𝛼4𝛽4 subunits:
𝛼 subunits contain enoyl-CoA hydratase and 𝛽-hydroxyacyl-CoA dehydrogenase activity
𝛽 subunits contain thiolase activity
the chemical logic of the 𝛽-oxidation sequence
The first three reactions convert the stable single bond between methylene groups to a much less stable C-C bond
The ketone function on the 𝛽 carbon (C-3) makes it a good target for nucleophilic attack
The terminal -CH2-CO-S-CoA is a good leaving group, facilitating breakage of the 𝛼-𝛽 bond
Also, it is a conserved reaction sequence similar to that of the citric acid cycle and the oxidation of isoleucine (leucine, valine)
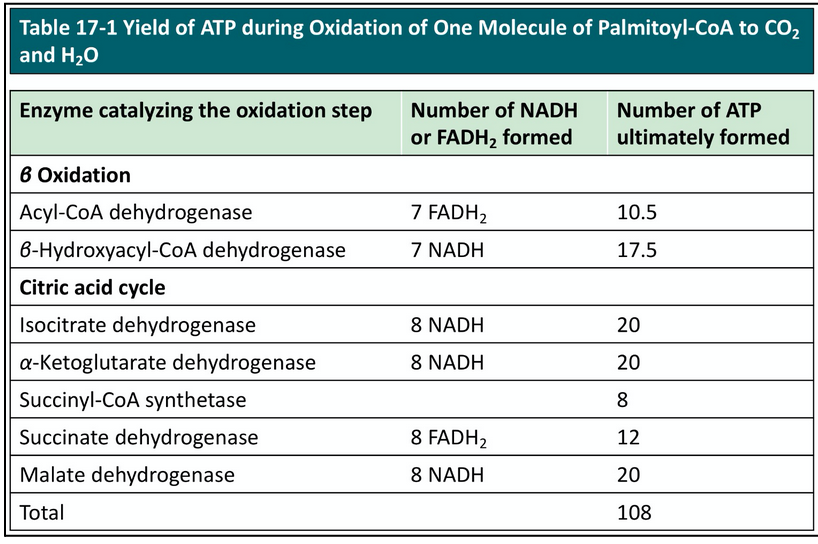
how much ATP per B-oxidation rounds and how much ATP per acetyl-CoA?
Each FADH2 donates a pair of electrons to ETF (electron-transferring flavoprotein) → generates 1.5 molecules of ATP
Each NADH donates a pair of electrons to the mitochodnrial NADH dehydrogenase → generates 2.5 molecules of ATP
In TOTAL, 4 ATP are formed for each pass through 𝛽 oxidation
PER acetyl-CoA → 10 ATP made
complete oxidation of palmitoyl-CoA ATP yield and number of FADH2/NADH
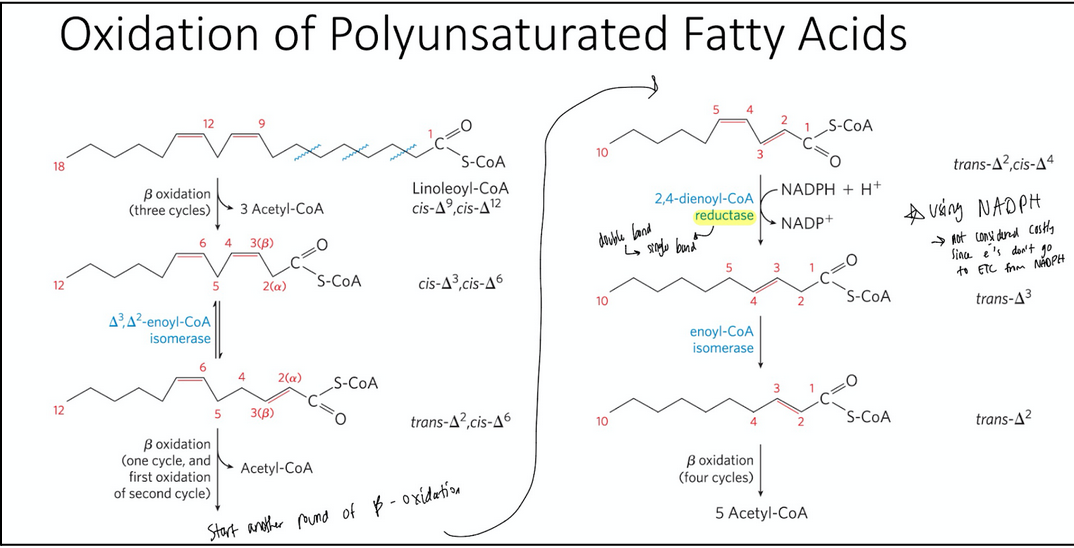
Oxidation of UNSATURATED fatty acids requires two additional steps
enoyl-CoA hydratase cannot catalyze the addition of H2O to a cis double bond
Oxidation of unsaturated fatty acids requires two additional enzymes:
enoyl-CoA isomerase (converts cis double bonds to trans; only this is needed for a monounsaturated fat)
2,4-dienoyl-CoA reductase (reduced cis double bonds; required because of the spacing between the double bonds in a PUFA)
oxidation of a monounsaturated vs. polyunsaturated fatty acid
Oxidation of a monounsaturated fatty acid requires an enoyl-CoA isomerase
In beta oxidation it was OHOT but now its just HOT, skips the first step, oxidation
Since you skip this step there is no reducing FAD → FADH2 so you lose 1.5 ATP; only 2.5 ATP from NADH step
∆3,∆2-enoyl-CoA isomerase = isomerizes the cis-∆3-enoyl-CoA to the trans-∆2-enoyl-CoA
Changes the double bond to an alpha beta double bond so it can be recognized
For polyunsaturated fatty acids it requires 1,2-dienoyl-CoA reductase
reductase converts double bond to single bond
uses NADPH —> not considered costly since electrons don’t go to ETC from NADPH
odd-number fatty acids
requires three extra reactions
Propionate (CH3-CH2-COO-) = three-carbon compounds formed by cattle and other ruminant animals during carbohydrate fermentation
Odd-number fatty acids are oxidized by the 𝛽-oxidation pathway to yield acetyl-CoA and a molecule of propionyl-CoA (when done with cycles of 𝛽-oxidation)
A 3 carbon molecule cannot enter TCA cycle
Odd numbered carbon chains undergo beta oxidation cycles until the last 5 carbons → the final round of beta oxidation produces 1 acetyl-CoA and 1 propionyl-CoA. The acetyl-CoA will generate 10 ATP and the propionyl-CoA yields 4 ATP
odd-number fatty acids: step 1 oxidation of propionyl-CoA
propionyl-CoA carboxylase = catalyzes the carboxylation of propionyl-CoA to form D-methylmalonyl-CoA
Requires the cofactor biotin
Requires ATP and bicarbonate
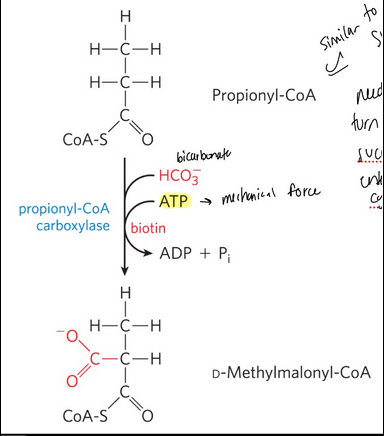
odd-number fatty acids: step 2 oxidation of propionyl-CoA
methylmalonyl-CoA epimerase = catalyzes the epimerization of D-methylmalonyl-CoA to its L stereoisomer
Swivel around (rotation) → requires enzyme
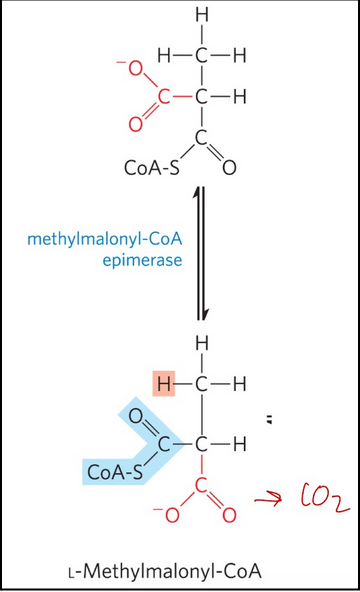
odd-number fatty acids: step 3 oxidation of propionyl-CoA
methylmalonyl-CoA mutase = catalyzes the intramolecular rearrangement of L-methylmalonyl-CoA to form succinyl-CoA (which can enter the citric acid cycle)
Requires 5’-deoxyadenosylcobalamin, or coenzyme B12, as its coenzyme
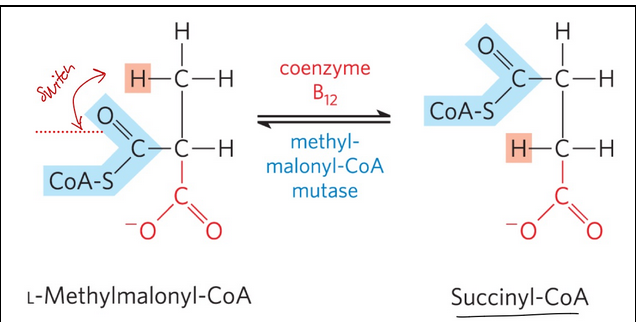
Do we gain anything from metabolism of odd-numbered carbon chain fatty acids?
Yes!
It costs 1 ATP to activate propionyl-CoA to generate D-methylmalonyl-CoA
But we produce 1 succinyl-CoA which will yield
1 GTP
1 FADH2
1 NADH
5 ATP equivalents but NET = 4 ATP
Need 1 of the ATPs to add CO2 (this was the first step in oxidation of propionyl-CoA)
each round of beta oxidation produces
Per round of OHOT 4 ATP are produced by the e-transport chain from the FADH2 and NADH produced
Each round of beta-oxidation produces 1 acetyl-CoA molecule and the last round produces 2* when even numbers of carbons. Each acetyl-CoA can turn the TCA cycle once producing 10 ATP equivalents
peroxisomes
organelles found in plants and animals
differences between the peroxisomal and mitochondrial pathways of lipid breakdown
In peroxisomes, the flavoprotein acyl-CoA oxidase that introduces the double bond passes electrons directly to O2, producing H2O2
The enzyme catalase cleaves H2O2 to H2O and O2
The peroxisomal system is much more active on very long chain fatty acids and branched-chain fatty acids since they have a hard time getting into the mitochondria
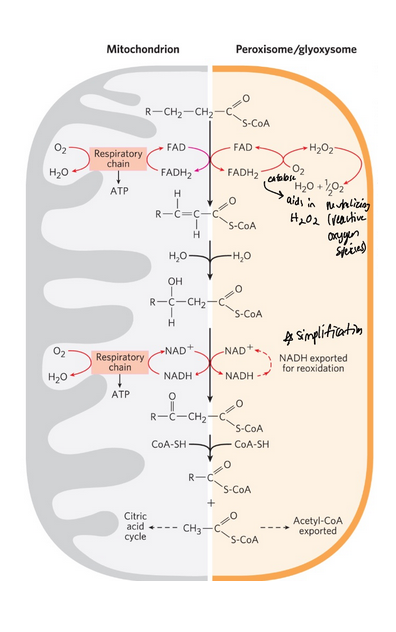
genetic defects in peroxisomal oxidation (2)
Zellwger syndrome = characterized by an inability to make peroxisomes
Individuals lack all metabolism related to peroxisomes
X-linked adrenoleukodystrophy (XALD) = characterized by the inability of peroxisomes to oxidize very long chain fatty acids
Due to the lack of a functional transporter in the peroxisomal membrane
Involves mutations in the ABCD1 transporter → which transports very long chain fatty acids into the peroxisome for oxidative breakdown
Accumulation of very long chain fatty acids in the blood plasma is a diagnostic hallmark of these disorders
Increasing the concentrations of these fats leads to neurological disorders including demyelination → motor impairment, memory loss and seizures
Bone marrow transplants and lentiviral therapies reduce oxidative stress due to lipid accumulation
Lorenzo’s oil restricts biosynthesis of very long chain fatty acids
genetic defects in fatty acyl-CoA dehydrogenases cause serious disease (1)
Medium-chain acyl-CoA dehydrogenase (MCAD) = acyl-CoA dehydrogenase isozyme that acts on fatty acid of 4-14 carbons
Individuals with two mutant MCAD alleles cannot oxidize fatty acids of 6-12 carbons
Symptoms include fatty liver, high blood levels of octanoic acid (8:0), coma and death
Longer chain breakdown still occurs (in peroxisomes then in mitochondria) but once tail is too short ie. medium chain length → NO beta oxidation occurs, build up in mitochondria of medium chain fatty acids
peroxisome disorders
Peroxisomes can also break down fatty acids in beta oxidation (and synthesize lipids too) and are responsible for the break down of the very long chain hydrocarbons and branched fatty acids
Rare genetic linked disorders are characterized by high blood serum levels of long chain fatty acids -- which cause inflammation, complications with the neuron sheath and are fatally associated with dysregulation of peroxisomes
Mutations in the ABCD1 transporter cause disorders in importing long chain fatty acids into the peroxisome which causes X-linked ALD disorders with very far-reaching clinical presentations