Chapter 21: Alpha Carbon Chemistry
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Enol & Enolate Intermediates
reactions occur either through enol or enolate intermediates
*must have ⍺ carbon
enolates are more reactive than enols b/c the ⊖ charge on O
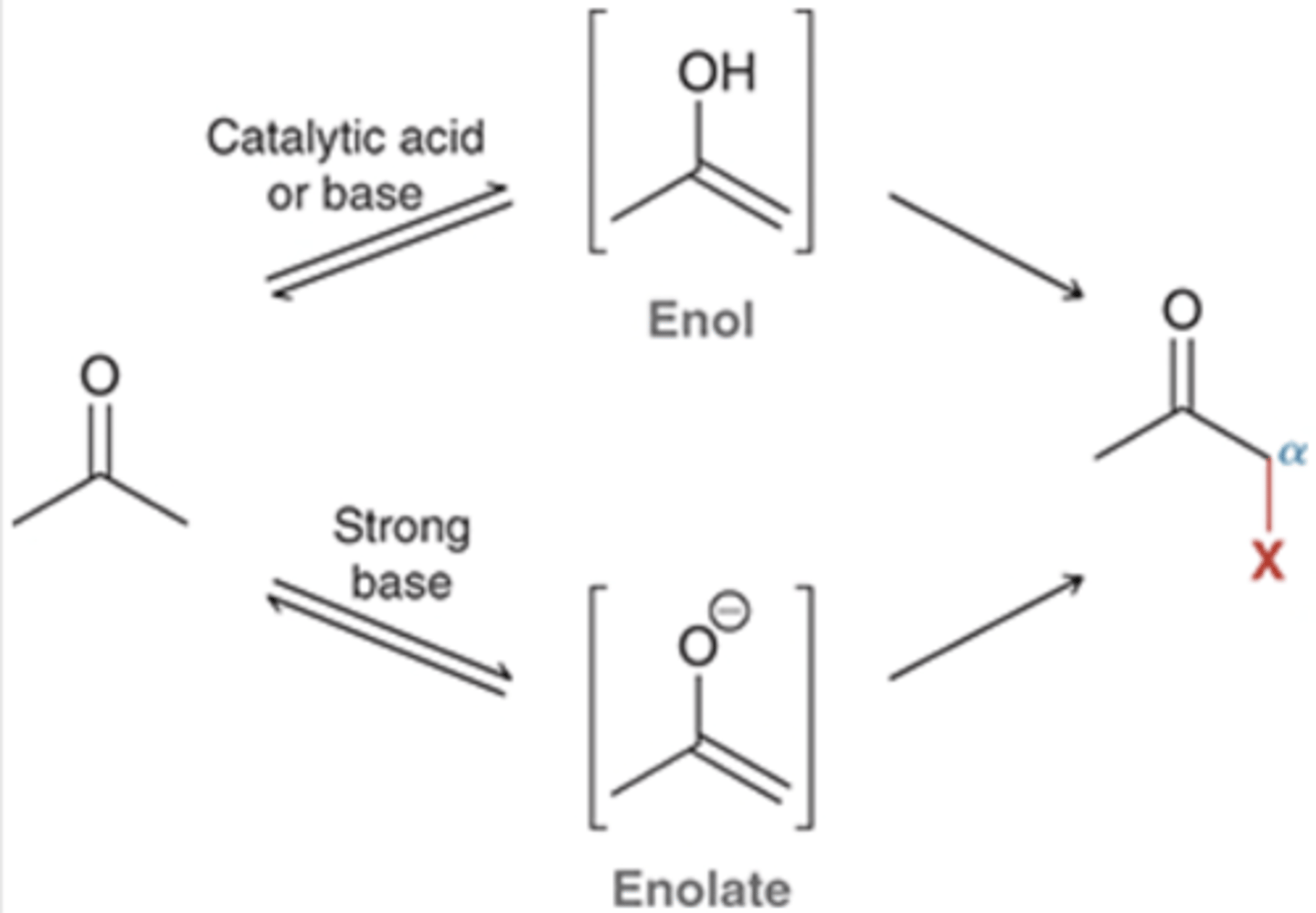
Enol Intermediates
in presence of catalytic base or acid
→ ketone exists in equilibrium w/ enol
ketone & enol are tautomers
(not resonance structures)
equilibrium favors ketone (99%) unless:
1) conjugated system → favors enol
2) intramolecular H-bond (like in diketone) → favors enol
enol is very reactive b/c its ⍺ carbon is very nucleophilic
(OH activates carbon)
Acid-Catalyzed Tautomerization
Starting Material: Ketone
Acidic Tautomerization → Enol
Reagents: H3O+
*assume tautomerization will occur if possible
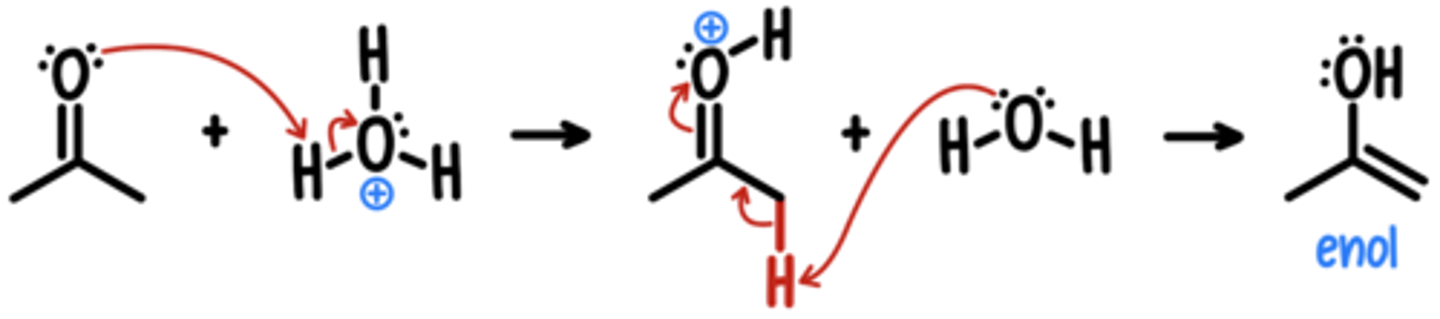
Base-Catalyzed Tautomerization
Starting Material: Ketone
Basic Tautomerization → Enol
Reagents: OH-
*assume tautomerization will occur if possible
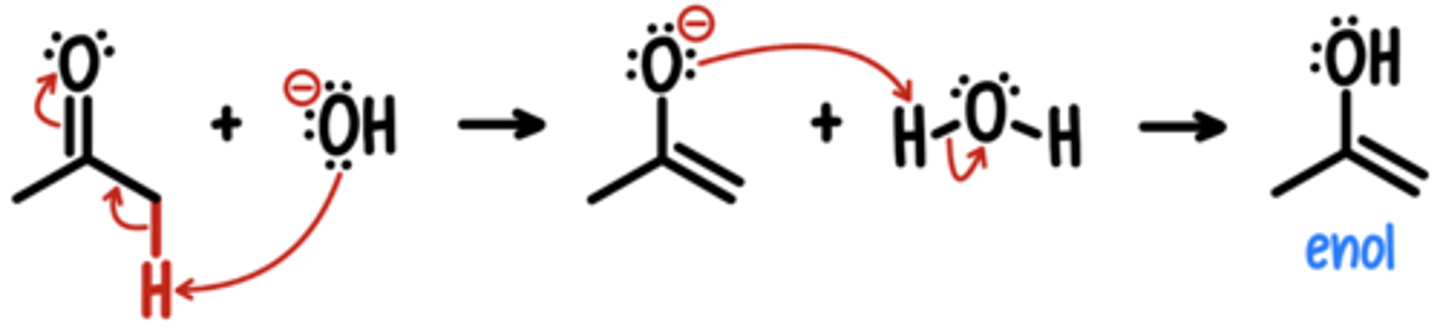
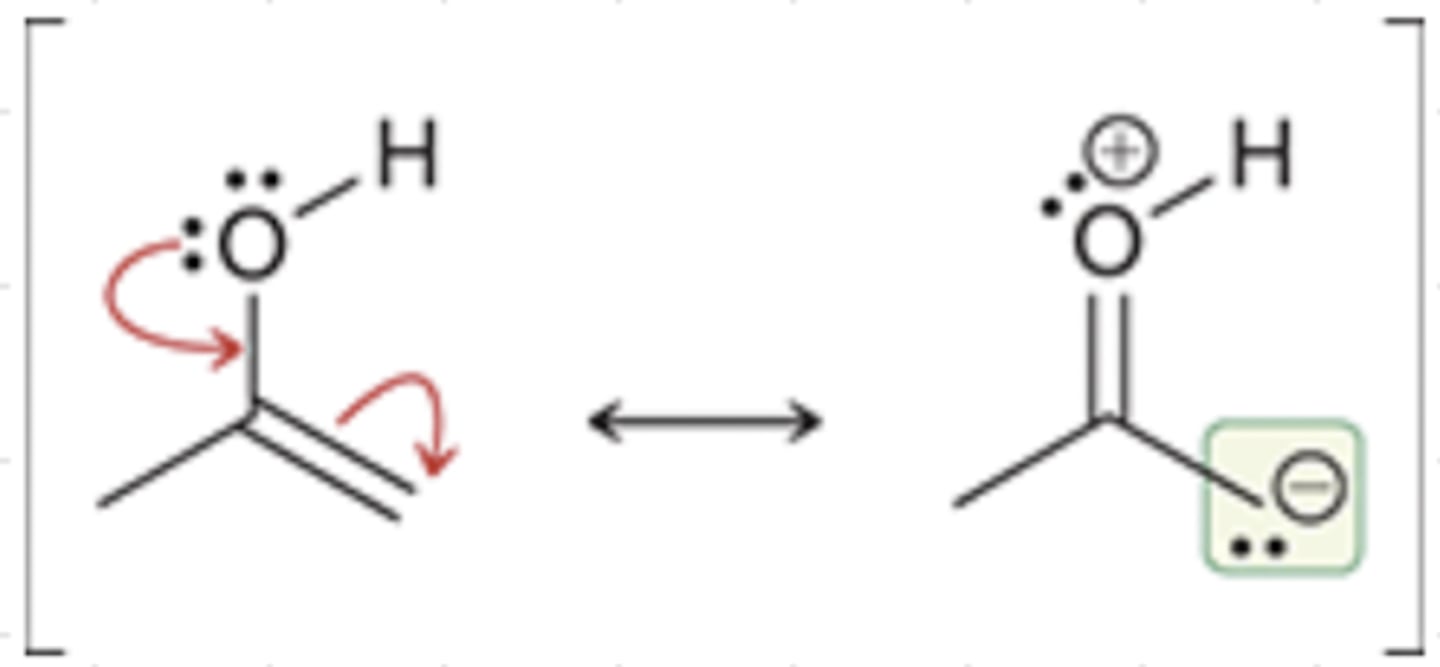
Enolate Intermediates
⍺ carbon of ketone treated w/ strong base → enolate intermediate
enolates have 2 nucleophilic sites: O & C
(C attacks are more common)
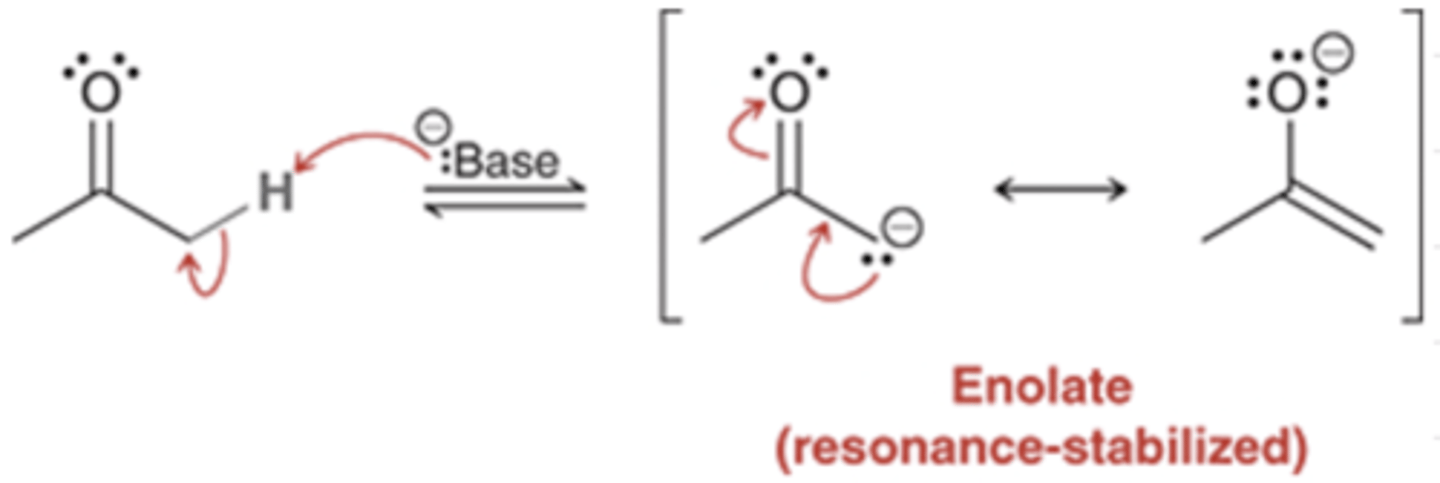
pKa Values Predicting Equilibrium Shift
similar pKa values (milder base)
→ both reactants & enolate present
strong base like NaH/LDA used
→ only enolate present (irreversible)
diketone reactant → mild base is sufficient to ensure irreversible enolate
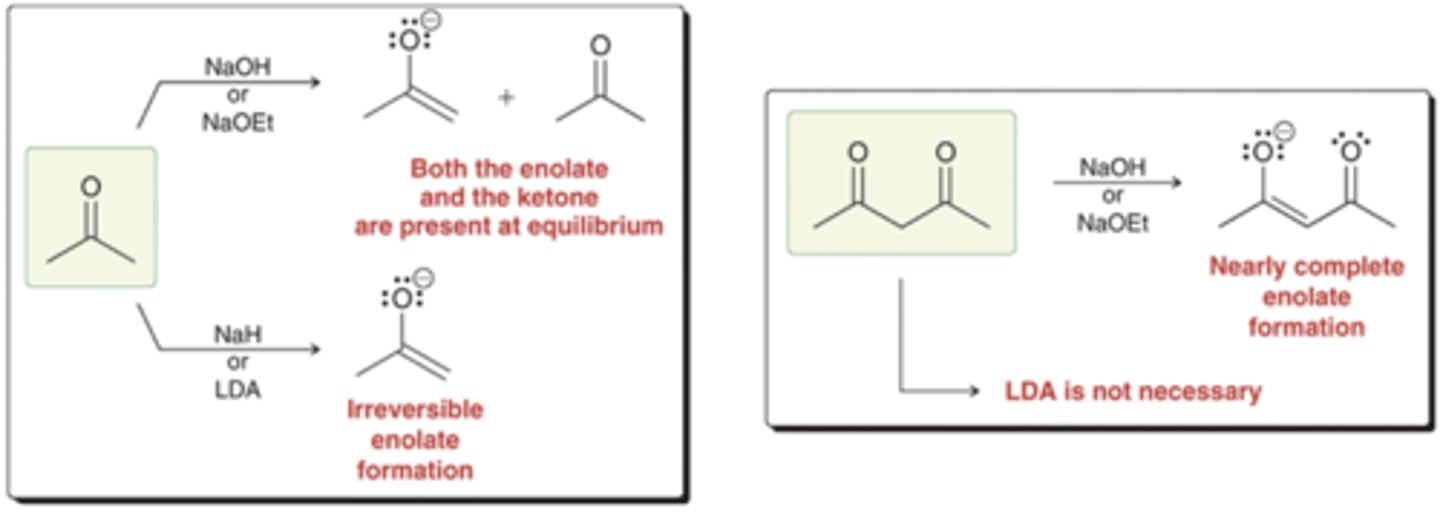
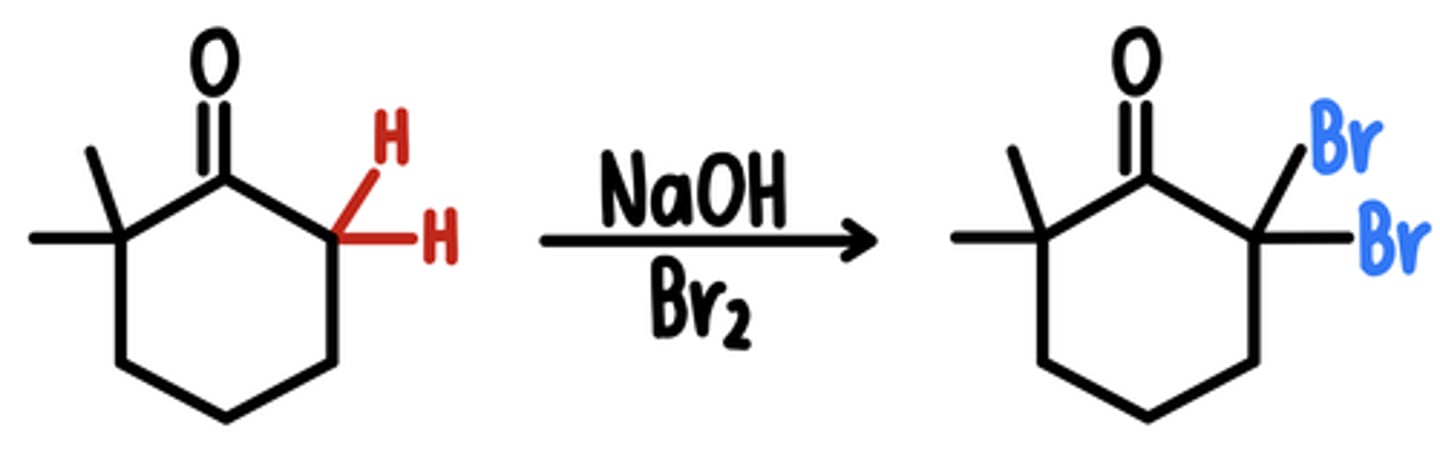
Alpha Halogenation in Basic Conditions
Starting Material: Aldehyde/Ketone
Basic Alpha Halogenation → all ⍺ protons replaced w/ Br
Reagents: NaOH, Br2
Intermediate: Enolate
*if unsymmetrical, Br added to more substituted side
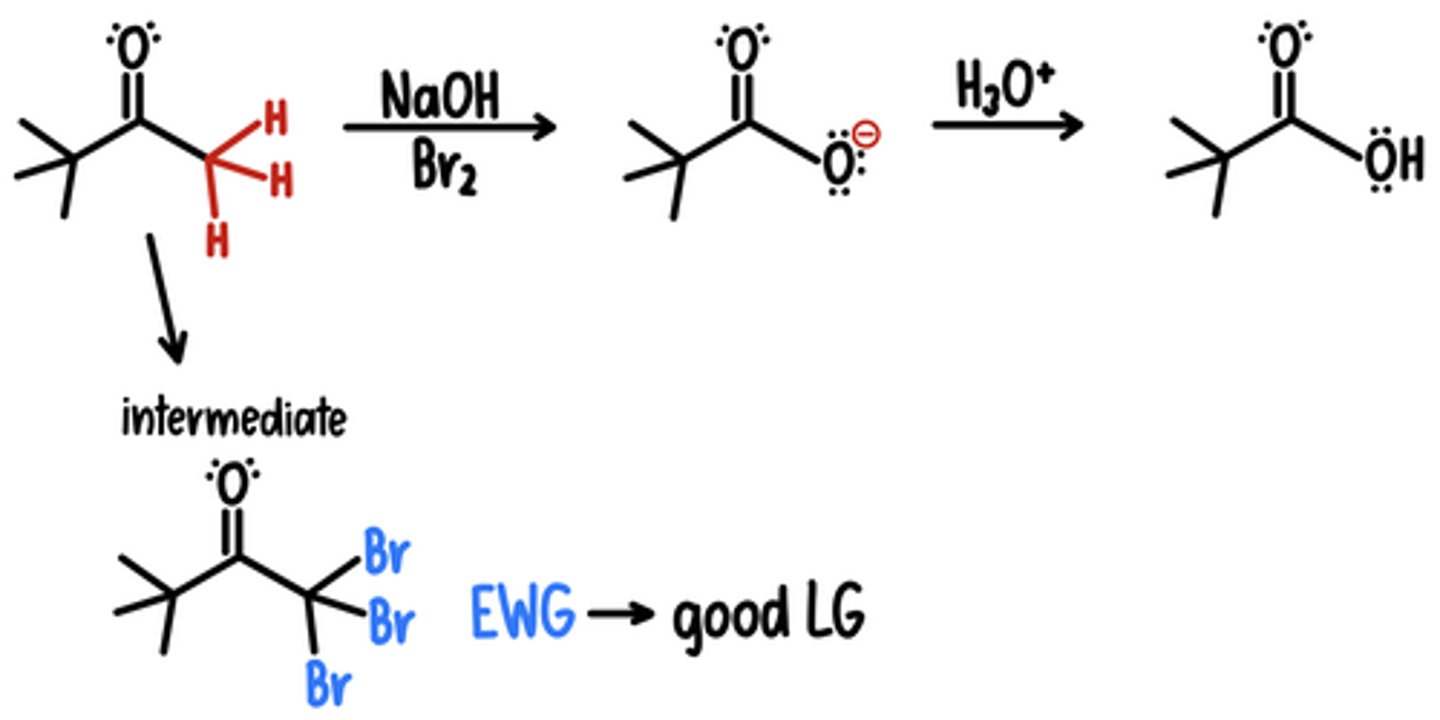
Haloform Reaction
Starting Material: Tribromomethyl
Haloform Reaction → Carboxylic Acid
Reagents: 1) NaOH, Br2 / 2) H3O+
Intermediate: -CBr3 (good LG)
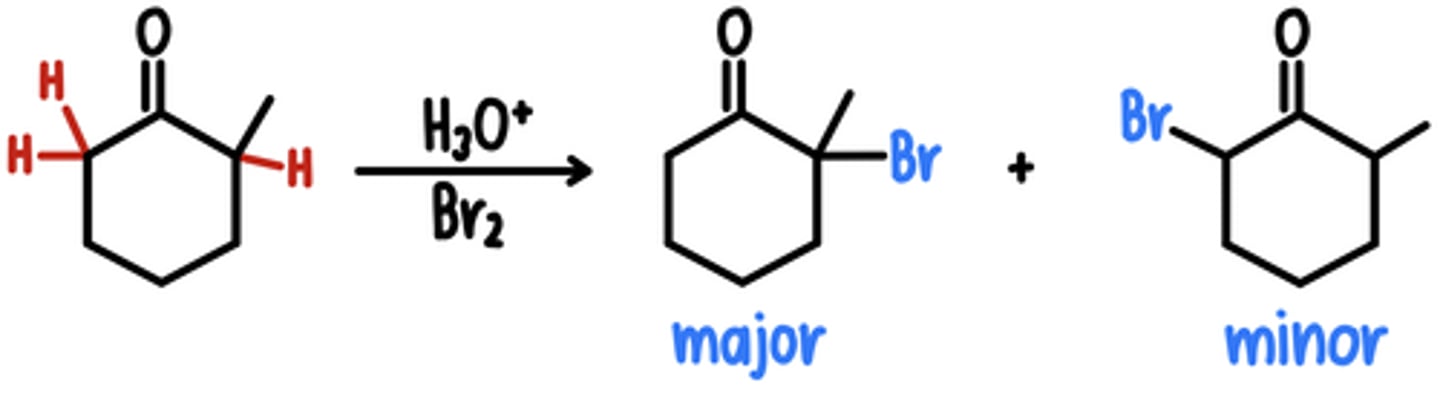
Alpha Halogenation in Acidic Conditions
Starting Material: Aldehyde/Ketone
Acidic Alpha Halogenation → 1 ⍺ proton replaced w/ Br
Reagents: H3O+, Br2
Intermediate: Enol
*if unsymmetrical, Br added to more substituted side
Aldol Addition Reaction
Starting Material: Aldehyde
Aldol Addition → ꞵ-Hydroxy Aldehyde/Ketone (OH at ꞵ)
Reagents: H3O+, Br2
Intermediate: Enolate
*reversible → retro-aldol reaction
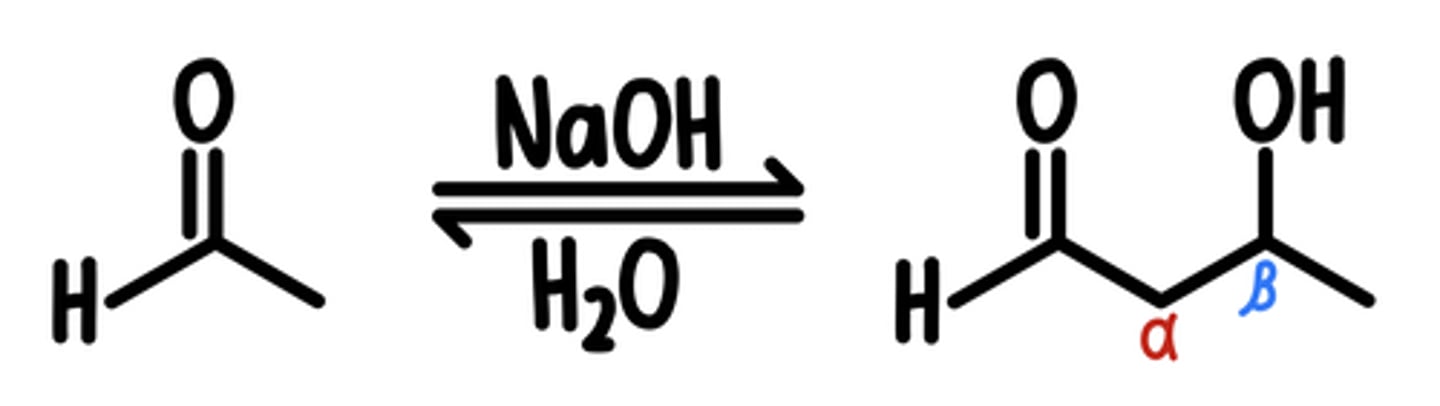
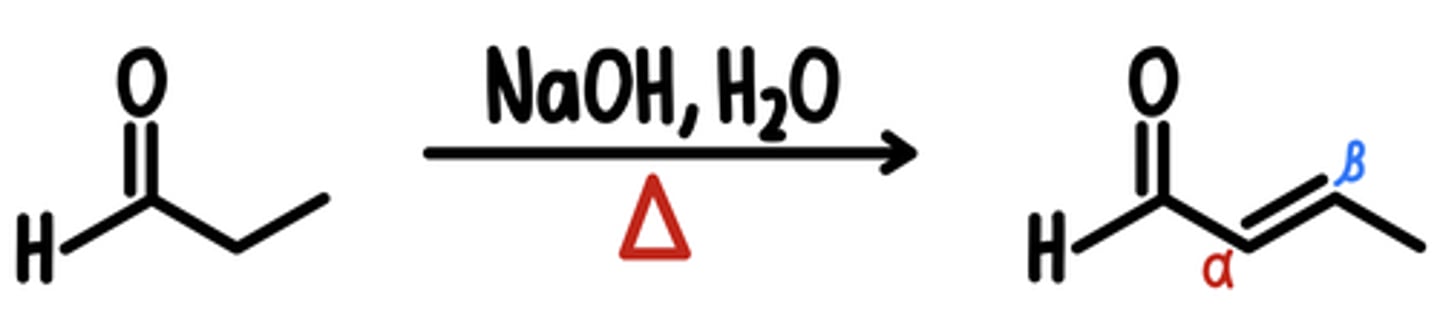
Aldol Condensation Reaction
aldol condensation = aldol addition + elimination
Starting Material: Aldehyde/Ketone
Aldol Condensation → ⍺,ꞵ-Unsaturated Aldehyde/Ketone
Reagents: NaOH, H2O, heat
*driving force → increased conjugation
Crossed Aldol Reaction
getting 1 product btwn different aldehydes/ketones
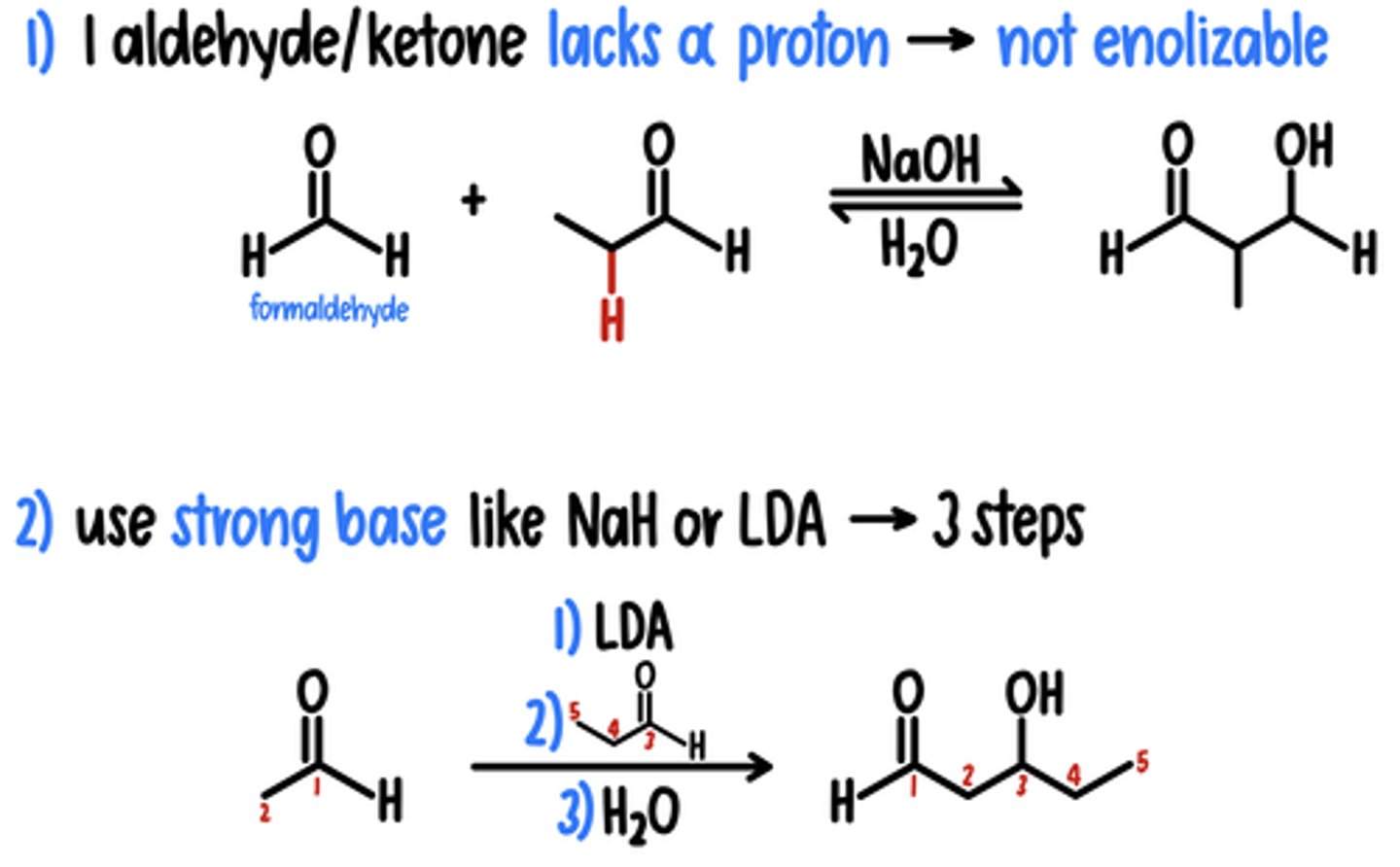
1) 1 Aldehyde/Ketone Lacks ⍺ Proton → Not Enolizable
Reagents: NaOH, H2O
2) Strong Base (NaH or LDA) → 3 Steps
Reagents: 1) NaH (or LDA) / 2) Aldehyde/Ketone / 3) H2O
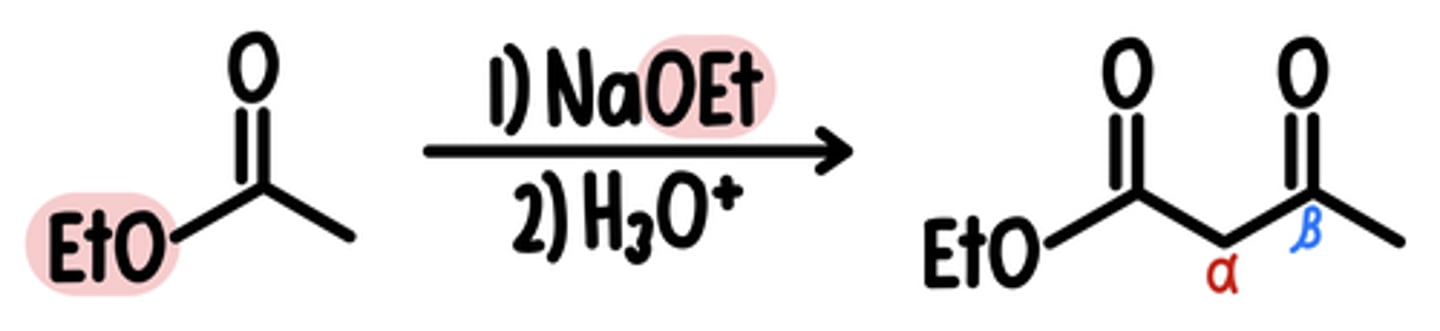
Claisen Condensation Reaction
Starting Material: Ester
Claisen Condensation → ꞵ-Keto Estr
Reagents: 1) NaOEt / 2) H3O+
Intermediate: Ester Enolate
*RO groups must be the same
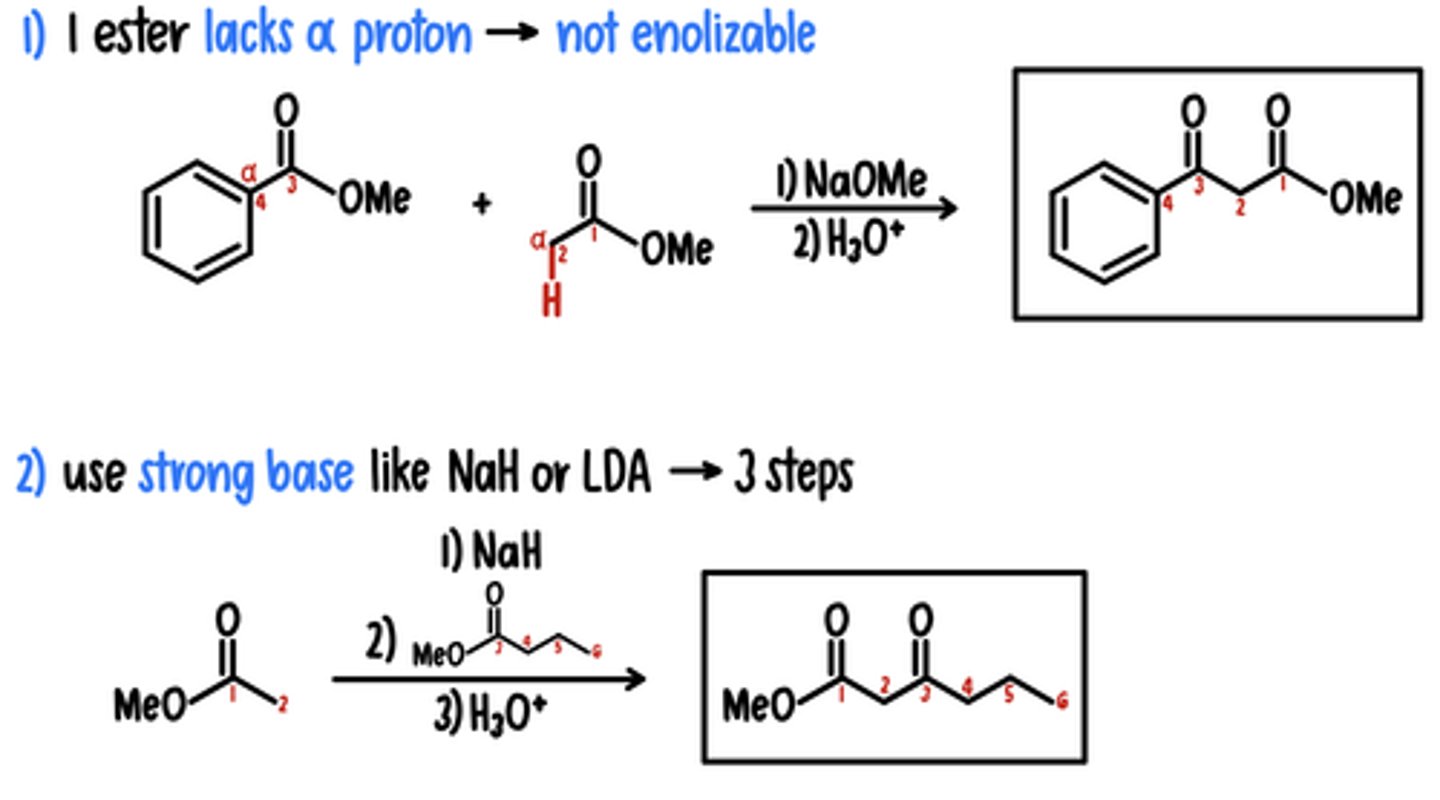
Crossed Claisen Condensation Reaction
getting 1 product btwn different esters
1) 1 Ester Lacks ⍺ Proton → Not Enolizable
Reagents: 1) NaOMe / 2) H3O+
2) Strong Base (NaH or LDA) → 3 Steps
Reagents: 1) NaH (or LDA) / 2) Ester / 3) H3O+
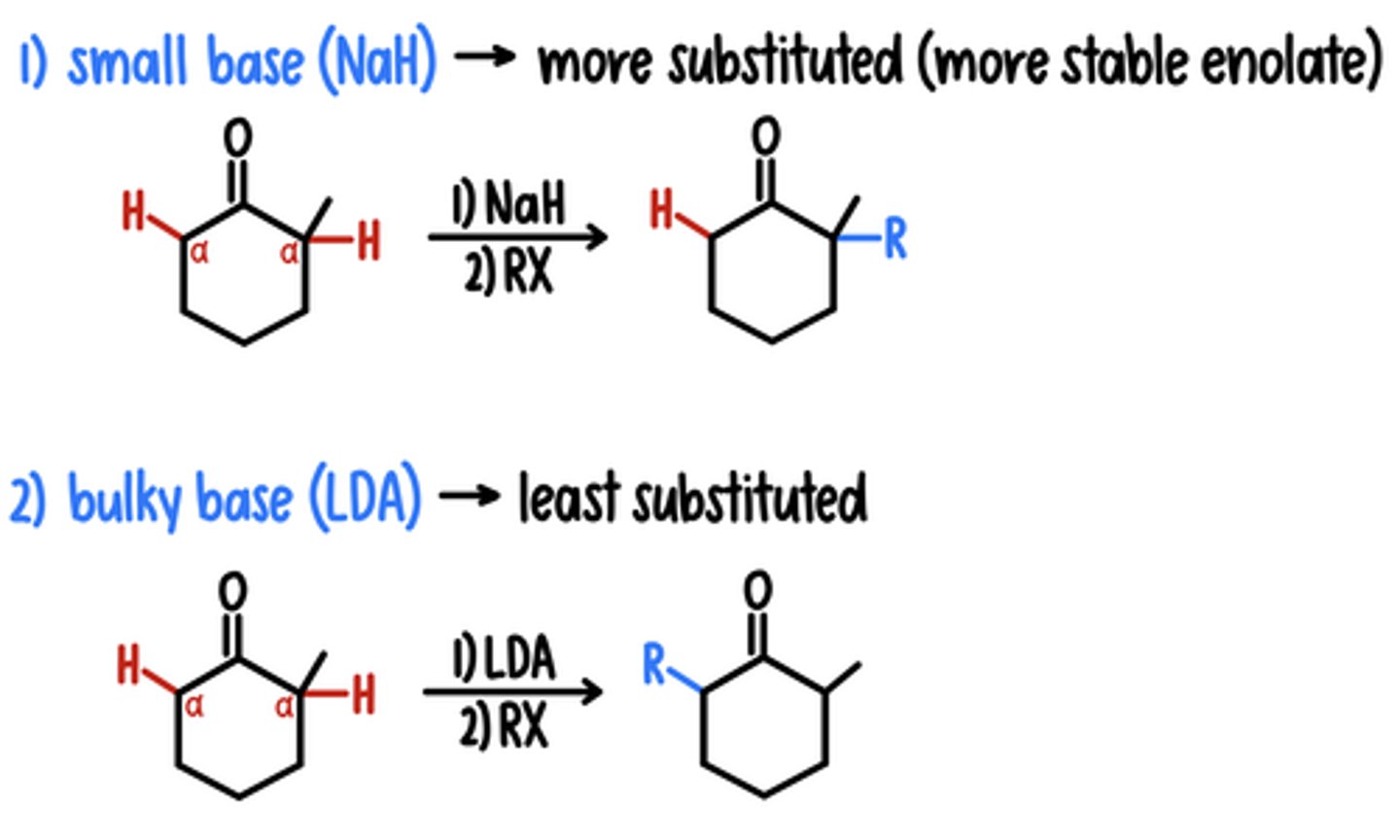
Alkylation of ⍺ Position
Starting Material: Ketone
1) Small Base (NaH) → R Added at More Substituted
Reagents: 1) NaH / 2) RX
2) Bulky Base (LDA) → R Added at Least Substituted
Reagents: 1) LDA / 2) RX
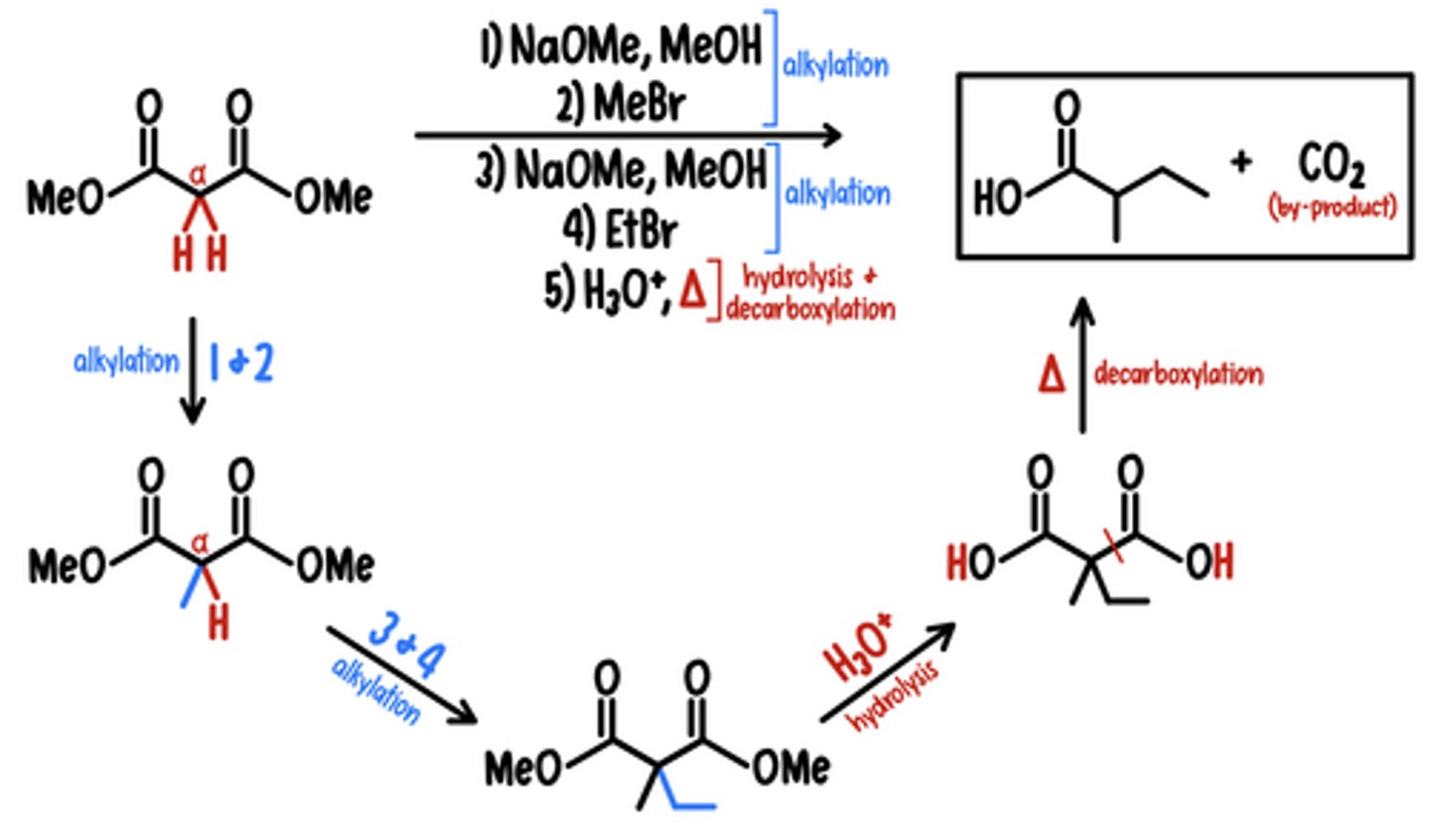
Malonic Ester Synthesis
Starting Material: Large Ester Compound
Malonic Ester Synthesis → Basic Carboxylic Acid
Reagents: 1) NaOMe, MeOH / 2) MeBr / 3) NaOMe, MeOH / 4) EtBr / 5) H3O+, heat
*forms CO2 by-product
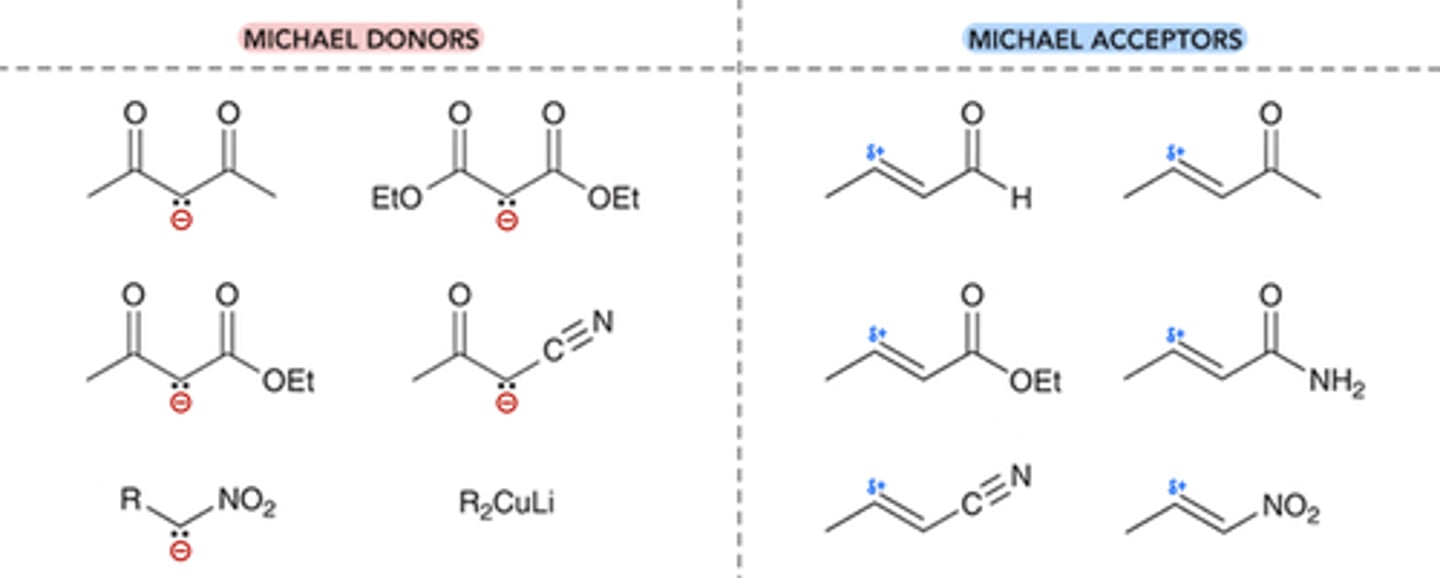
Michael Acceptors vs Donors
Michael Acceptor (E+) → ⍺,ꞵ-Unsaturated Compound
*unique reactivity: 2 E+ positions
Michael Donor (Nuc-) → Enolate Ion
*large & weak compound (favors attack at ꞵ carbon)
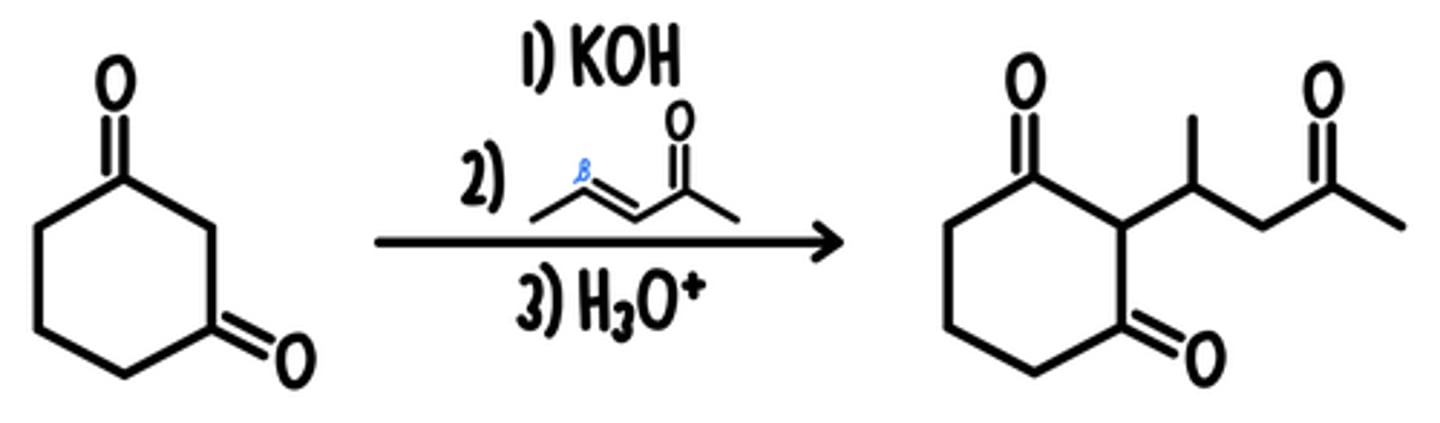
Michael Synthesis
Starting Material: Michael Donor (Nuc-)
Michael Synthesis → Combines Donor + Acceptor
Reagents: 1) KOH / 2) Michael Acceptor (E+) / 3) H3O+
*keto-enol tautomerization occurs after product is formed