Topic 2/8 - Energy and Aerobic Metabolism
0.0(0)
Card Sorting
1/204
Earn XP
Description and Tags
Last updated 3:18 AM on 11/17/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
205 Terms
1
New cards
Which defining characteristics of life are low energy?
cellular organization (make organelles), homeostasis (in optimal environments), reproduction (when not reproducing), heredity (cell life)
2
New cards
Which defining characteristics of life are high energy?
growth and metabolism, homeostasis (in nonoptimal environments), reproduction (when reproducing)
3
New cards
What is the solar constant?
the amount of energy the earth receives from the sun, 51% is absorbed by land and oceans while 49% is reflected back into space
4
New cards
What happens to the solar energy that is absorbed by the Earth?
used to maintain the temperature of the land, oceans and air (no sun = ice age), captured by autotrophs and converted into chemical bond energy (base of food webs)
5
New cards
What is the Urey-Miller experiment?
simulation of conditions on early Earth testing the idea that life, or more specifically organic molecules, could have formed by simple chemical reactions
6
New cards
What is the accepted theory of early evolution?
1. primordial soup
2. lightning (energy)
3. urey-miller experiment
4. abiogenesis
2. lightning (energy)
3. urey-miller experiment
4. abiogenesis
7
New cards
What is abiogenesis?
first life forms generated were very simple and through a gradual process became increasingly complex
8
New cards
What was the early atmosphere like?
anaerobic: no O2, mostly CO2, some N2
9
New cards
What were the first organisms to evolve?
anaerobic heterotrophs: able to get a small amount of energy from glucose without O2 (breakdown chemicals into nutrients with fewer enzymes)
early autotrophs: able to make their own molecules to get energy (H2S, then H2O)
true autotrophs: able to undergo photosynthesis (use oxygen)
early autotrophs: able to make their own molecules to get energy (H2S, then H2O)
true autotrophs: able to undergo photosynthesis (use oxygen)
10
New cards
What is the modern atmosphere like?
aerobic: lots of O2, mostly N2 and some CO2
11
New cards
What is aerobic metabolism?
metabolism in the presence of oxygen used by aerobic heterotrophs (glucose is eaten, not made)
12
New cards
Is evolution goal oriented?
no, it is random (due to mutations). it will never start over from scratch and will simply build on existing pathways (its inefficient but nothing else exists)
13
New cards
What is the energy currency of life?
ATP (adenosine triphosphate) s the preferred activation energy
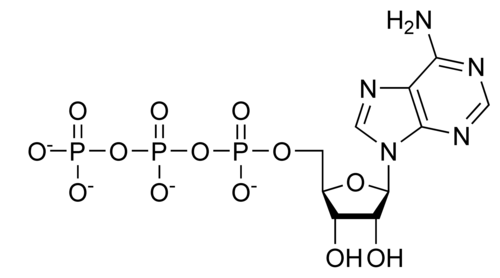
14
New cards
How is ATP formed?
energy from the sun is stored as chemical energy in the bonds of glucose, cellular organisation converts the stored chemical energy into usable energy in high energy bonds of ATP
15
New cards
Why are the bonds in the phosphate group on ATP considered to be high energy bonds?
O charge on PO4 is negative, and it is harder to put negative ions together
16
New cards
What is the reaction for the hydrolysis of ATP?
the reaction of ATP and water yields ADP and inorganic phosphate (Pi - phosphate not attached to carbon) and releases energy (exothermic)
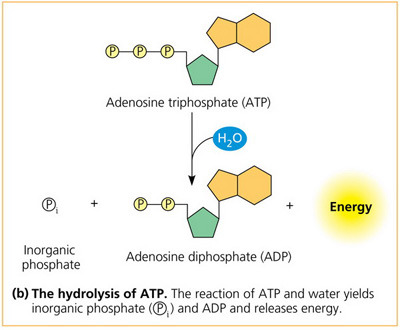
17
New cards
Is the hydrolysis of ATP exothermic or endothermic, and why?
highly exothermic as the high-energy bonds release energy when broken
18
New cards
Is the synthesis of ADP to ATP exothermic or endothermic, and why?
highly endothermic as putting 2 functional groups with like charges (that repel) together takes a lot of energy
19
New cards
How much energy is released when a high energy bond breaks?
7.3 kcal/mol of energy per bond (31 kJ/mol)
20
New cards
What is the activation energy of the average biochemical reaction?
3.5 kcal/mol (15.5 kJ/mol)
21
New cards
What is AMP?
adenosine monophosphate or nucleic acids (RNA/DNA)
22
New cards
What are coupled reactions?
when two reactions occur in close proximity, so the energy released by the exothermic reaction can be used to provide the activation energy needed for the endothermic reaction
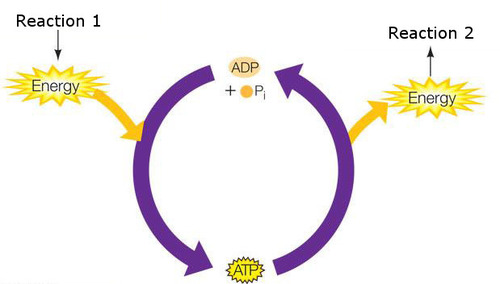
23
New cards
What was the first way to make ATP without O2?
substrate-level phosphorylation (SLP)
24
New cards
What is substrate-level phosphorylation?
direct transfer of a phosphate group from an organic substrate to ADP by an enzyme (usually a kinase); this process occurs during glycolysis and the kreb cycle, but generates much less ATP during cellular respiration than oxidative phosphorylation
25
New cards
How is ATP made when there is O2?
chemiosmosis or oxidative phosphorylation
26
New cards
What is oxidative phosphorylation?
a series of reactions in which electrons are transferred from NADH and FADH2 (the molecules are oxidized) to different electron acceptors (such as oxygen) to drive the phosphorylation of ADP to ATP; this process occurs through an electron transport chain that establish a proton gradient, followed by chemiosmosis that produces ATP
27
New cards
What does the proton pump do?
creates an electrochemical concentration gradient across the inner mitochondrial membrane by actively transports hydrogen ions (H+) out of the cell
28
New cards
How many protons (hydrogen ions) go through ATP synthase to create 1 ATP?
3 (correct: 3.5)
29
New cards
What is ATP synthase?
an enzyme/channel protein that helps the ions cross the inner mitochondrial membrane
30
New cards
What is the proton motive force?
the force provided by a transmembrane hydrogen ion gradient, creating kinetic energy within ATP synthase that is converted into the chemical energy in the high energy bond in ATP
31
New cards
Where does SLP occur? Does it occur during aerobic or anaerobic metabolism?
cytoplasm for glycolysis and mitochondrial matrix for krebs cycle (eukaryotes), during aerobic or anaerobic respiration
32
New cards
Where does oxidative phosphorylation occur? Does it occur during aerobic or anaerobic metabolism?
inner mitochondrial membrane during aerobic respiration (for animals)
33
New cards
What is cellular respiration?
process by which all cells release the energy from the bonds of glucose into usable energy (ATP)
34
New cards
What are the 3 main stages of cellular respiration? Do they make ATP directly or indirectly?
glycolysis (makes ATP directly), oxidation of pyruvate (makes ATP indirectly), krebs cycle (makes ATP directly)
35
New cards
What is the equation for cellular respiration?
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + ATP energy
36
New cards
What is glycolysis?
first stage of cellular respiration, occurs in cytoplasm, occurs with or without oxygen
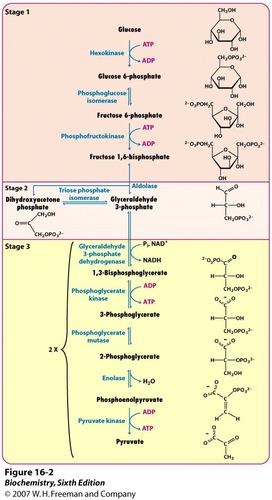
37
New cards
What are the 3 pairs of isomers in glycolysis?
glucose/fructose, DHAP/G3P, 3PG/2PG
38
New cards
What are the 2 exothermic reactions in glycolysis?
ATP losing PO4 to become ADP in reactions 1 and 3
39
New cards
How many ATP are used and produced in the energy investment phase of glycolysis?
2 ATP used, 0 are made
40
New cards
What is the final product of the energy investment phase of glycolysis?
2 molecules of G3P
41
New cards
Where is the redox reaction in glycolysis?
reaction 6 where G3P becomes 1,3-BPG
42
New cards
What are 2 examples of SLP in glycolysis?
reaction 7 and 10 where ADP becomes ATP
43
New cards
What does phosphorylation accomplish?
adding phosphate group (via kinase) makes new molecule unstable, so another reaction will occur soon after
44
New cards
What is the most common hydrogen/electron carrier? What does it become?
NAD+ (nicotinamide adenine dinucleotide) becomes NADH + H+
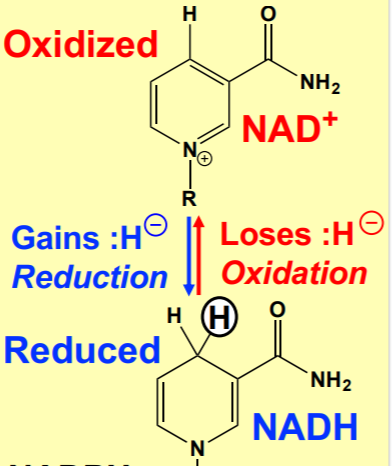
45
New cards
How much energy is in the bonds of glucose?
686 kcal/mol
46
New cards
How much usable energy has been extracted during glycolysis?
from 2 ATP (2 high energy bonds x 7.3 kcal/mol = 14.6 kcal/mol)
energy yield in glycolysis/chemical potential energy in glucose = 14.6/686 = 2%
energy yield in glycolysis/chemical potential energy in glucose = 14.6/686 = 2%
47
New cards
How much ATP is produced in glycolysis?
4 ATP, 2 are used so net 2 ATP
48
New cards
Where does the chemical potential energy in glucose go in glycolysis?
2% in ATP, 98% in bonds of pyruvate molecules
49
New cards
If glycolysis is so inefficient, why is it still around?
it works despite being inefficient so that's all there is: evolution
50
New cards
What are the limitations of glycolysis?
glycolysis will eventually convert all NAD+ to NADH unless there is some way to remove the electrons; if the cell runs out of NAD+, glycolysis will stop and the cell will run out of energy
51
New cards
What are the 2 ways to recycle NADH (after glycolysis)? Which evolved first and why?
fermentation (evolved first since it does not require oxygen) and oxidative respiration (evolved later since it requires oxygen)
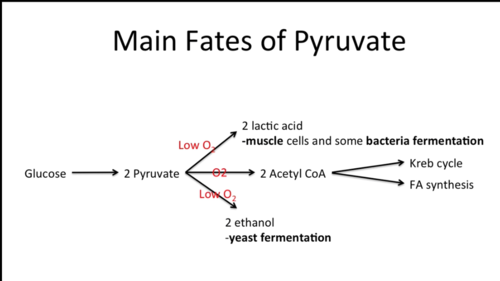
52
New cards
What is fermentation?
an ATP-generating process in which organic molecules act as both donors and acceptors of electrons, works under both aerobic and anaerobic conditions; another organic molecule removes the hydrogen (electrons) from NADH to recycle the NAD+
53
New cards
What is ethanol fermentation?
performed by yeast cells or bacteria; conversion of pyruvate to ethanol under aerobic or anaerobic conditions; used in baking and alcohol brewing
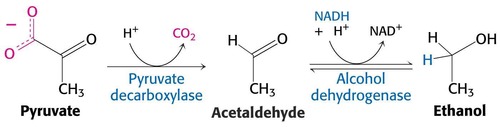
54
New cards
What is lactic acid fermentation?
performed by animal cells, some bacteria (lactobacilli) and fungi when deprived of O2; occurs under anaerobic conditions; catalyzed by lactate dehydrogenase
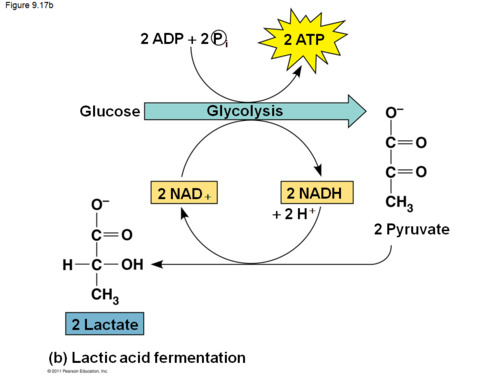
55
New cards
How is lactic acid produced in the body?
when the body cannot provide enough oxygen to the muscle cell, the body builds up lactic acid, causing fatigue and cramping; this lactic acid is then carried to the liver and converted back to pyruvate
56
New cards
What is oxidative respiration?
occurs under aerobic conditions in the mitochondria; pyruvate is oxidized and oxidative respiration continues
57
New cards
What is oxidation of pyruvate?
second stage of aerobic metabolism aka link reaction; catalyzed by pyruvate dehydrogenase; 3 main tasks: decarboxylation, oxidation of pyruvate to create acetyl group, addition of co-enzyme A
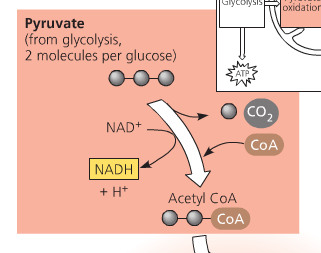
58
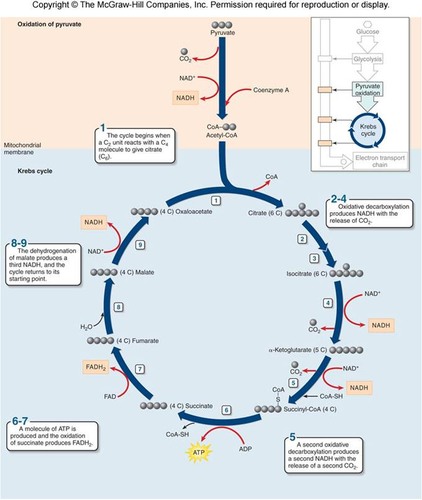
New cards
What is the Krebs cycle (citric acid cycle)?
third stage of aerobic metabolism; first reaction produces citrate/citric acid; cycle beginning with oxaloacetate (4C molecule that is regenerated); entry point for other metabolic processes (proteins, fats, lipids are turned into acetyl groups and enter Krebs cycle when digested)
59
New cards
How many times will the cell go through the Krebs cycle for each molecule of glucose? How many ATP are produced per molecule of glucose?
2 times, 1 ATP by SLP each time (2 ATP total)
60
New cards
Where are the decarboxylation reactions in the Krebs cycle?
reactions 3 and 4
61
New cards
Where are the redox reactions in the Krebs cycle?
reactions 3, 4, 6, 8
62
New cards
What is the most exothermic reaction in the Krebs cycle?
succinyl CoA --> succinate (reaction 5)
63
New cards
What are the two electron carriers in the Krebs cycle?
NAD+, FAD
64
New cards
What is the pair of isomers in the Krebs cycle?
citrate/isocitrate
65
New cards
What are NAD+ and FAD?
co-enzymes or electron carriers with high electron affinity so they removed hydrogen atoms and their electrons to oxidize other molecules and create reactive intermediates
66
New cards
What is NAD+/NADH? Where is it found?
(nicotinamide adenine dinucleotide) found in the cytoplasm and mitochondria, cannot cross the mitochondrial membrane
67
New cards
What is FAD/FADH2? Where is it from?
(flavin adenine dinucleotide), only found in mitochondria
68
New cards
Where do electron carriers NAD+/FAD carry electrons?
from electron transport chain to a series of proton pumps
69
New cards
What is the cristae of the mitochondria? What occurs here?
folds of the inner membrane, electron transfer chain and chemiosmosis occur here
70
New cards
How are electrons from NADH passed to FAD inside the mitochondria?
electrons from NADH can enter the mitochondrial ETC from reducing DHAP to G3P (oxidized intermediate); G3P is reoxidized by electron transfer to an FAD prosthetic group in membrane bound G3P dehydrogenase; electrons then enter the ETC
71
New cards
Why is it important for electrons but not NAD+ to pass the mitochondrial membrane?
maintains supply of NAD+
72
New cards
What is the electron transport chain?
a cluster of membrane proteins (cytochrome complexes) arranged in order of increasing electronegativity along the inner mitochondrial membrane; receive electrons from NADH/FADH2 and pass them along until they reach oxygen, the final electron acceptor
73
New cards
How many electrons are made in the electron transport chain?
2 electrons made
74
New cards
What happens there is no O2 in the electron transport chain?
if there was no O2 present in the mitochondria, the electrons could not be removed from the system, and the entire ETC would back up and stop (electrons would not be removed from NADH/FADH2); mitochondria would be unable to generate new ATP this way, and the cell would ultimately die from lack of energy
75
New cards
What is one of the products of cellular respiration made in the ETC?
H2O
76
New cards
What are the names of all complexes in the ETC?
complex 1: NADH reductase complex (e- from NADH to Q)
Q: ubiquinone, motile e- carrier (e- from NADH reductase or FADH2)
complex 2: cytochrome B complex (e- from Q to cyt. C)
cytochrome C: motile e- carrier
complex 3: cytochrome oxidase complex (e- from cyt. C to O2 making H2O)
Q: ubiquinone, motile e- carrier (e- from NADH reductase or FADH2)
complex 2: cytochrome B complex (e- from Q to cyt. C)
cytochrome C: motile e- carrier
complex 3: cytochrome oxidase complex (e- from cyt. C to O2 making H2O)
77
New cards
How many protons does each cytochrome complex pump out?
3-4 protons
78
New cards
How are the ETC and ATP synthase related?
electrons are passed along driven by a drop in free energy, energy lost pumps protons actively against concentration gradient; this creates huge concentration gradient; ATP synthase molecules create 1 ATP for every 3H+
79
New cards
Does the ETC make ATP?
not directly, but indirectly helps by creating the concentration gradient where 3H+ then make 1 ATP in chemiosmosis
80
New cards
How are triglycerides metabolized?
hydrolyzed by lipases into glycerol and fatty acids (have tons of energy/gram)
81
New cards
How is glycerol metabolized?
can be converted into glucose via gluconeogenesis (glycerol+glycerol=glucose)
82
New cards
How are fatty acids metabolized?
transported into mitochondrial matrix to undergo B-oxidation (broken into acetyl groups), then enter Krebs cycle (each cleavage uses 1 ATP and produces 3 NADH and 1 FADH2)
83
New cards
What is B-oxidation?
first step in fatty acid oxidation, in which fatty acids are broken into separate two carbon units of acetic acid, each of which is then converted to acetyl CoA
84
New cards
How much ATP is lost and gained from metabolizing lipids?
loss: - (number of acetyl groups)
gain: 11 x (number of acetyl groups)
gain: 11 x (number of acetyl groups)
85
New cards
How are monosaccharides metabolized?
aerobic metabolism
86
New cards
How is starch metabolized?
hydrolyzed by amylase into monosaccharides, then aerobic metabolism
87
New cards
How is glycogen metabolized?
removed from the liver and muscle cells to undergo glycogenolysis (break apart glycogen) to increase blood glucose
88
New cards
How is excess glucose metabolized?
converted to glycogen via glycogenesis (glucose to glycogen) and stored in the liver
89
New cards
How are polypeptides metabolized?
hydrolyzed by pepsin in stomach (main enzyme that breaks peptides into amino acids)
90
New cards
What is deamination?
all NH3 (amine groups) are removed from proteins are excreted as urea
91
New cards
Where do the remaining carbon skeletons from amino acids, sugars and nitrogenous bases enter aerobic metabolism?
can become acetyl CoA (krebs cycle) or pyruvate
92
New cards
How are nucleic acids metabolized?
negligible digestion; hydrolyzed into nucleotides which are usually recycled into new nucleotides but can be used as an energy source if absolutely necessary
93
New cards
ATP determines the fate of acetyl groups... What happens if there is low ATP?
acetyl groups are picked up by CoA and enter cellular respiration
94
New cards
ATP determines the fate of acetyl groups... What happens if there is high ATP?
acetyl groups are picked up by CoA that converts them into fatty acids for storage; phosphofructokinase is inhibited (adds P to fructose-6-phosphate)
95
New cards
What happens if there is high NADH in cellular respiration?
non-competitive inhibitor of pyruvate decarboxylase
96
New cards
What was Earth like before photosynthesis?
it was a reducing environment (gain electrons)... less than 2% oxygen
97
New cards
Did photosynthesis or cellular respiration evolve first? Why?
photosynthesis happened first since it doesn't require oxygen, and cellular respiration requires oxygen
98
New cards
What started photosynthesis?
cyanobacteria started photosynthesizing 2.5 bya, oxygen was a byproduct of the photolysis of water (breakdown of molecules by light)
99
New cards
What time period did oxygen levels rise?
750 mya to present - oxygen levels have risen from 2% to roughly 20% because of photosynthesis
100
New cards
What are the implication of oxygen production?
1. oxygen production led to the formation of the ozone layer, this made Earth livable since it reduced UV exposure allowing more life to survive and evolve
2. oxygen in the atmosphere led to oxidized compounds such as iron oxides that have formed rock depositions on the ocean floor
2. oxygen in the atmosphere led to oxidized compounds such as iron oxides that have formed rock depositions on the ocean floor