lec6: Cell Death and Signalling
1/61
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
Cell death is a natural process T or f
True
Cell death is necessary for
Homeostasis, development and immune function
Most cell death occur through a process of ……………
programmed cell death
Programmed cell death is responsible for
balancing cell proliferation and maintaining constant cell numbers in tissues undergoing cell turnover.
The defense mechanism by cell death helpsin
preventing the accumlation of dead or unnecessary cells that could lead to disease
virus infected cells to limit the spread of viruses
DNA damage to eliminate cells with potentially harmful mutations that might lead to cancer
Programmed cell death can lead to diseases and tissue damage t or f
false, excessive or unregulated cell death
Examples of unregulated cell death
cancer: mutations in genes that regulate cell death
neurodegenrative: excessive death of neurons
ischemic heart disease: blood supply is blocked to the heart, causing necrotic cell death (infarction)
Autoimmune disease: ex:rheumatoid arthiritis, immune system attacks health cells, leading to excessive cell death
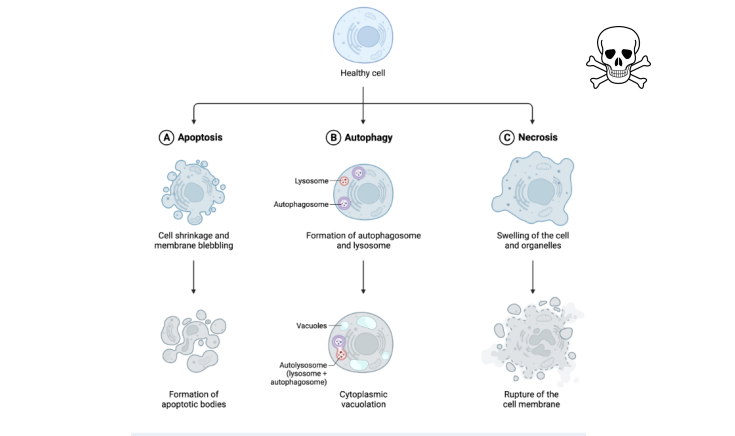
Major types of cell death
Apoptosis
Autophagy
Necrosis
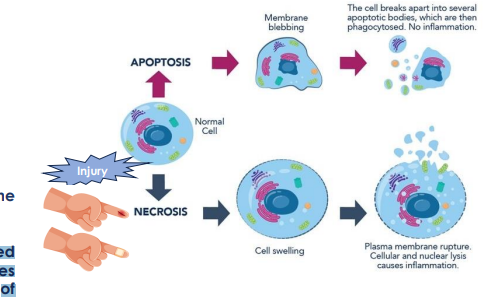
Apoptosis
programmed cell death
chromosomal DNA fragmentation, chromatin condensation, break up of nucleaus to small pieces, cell shriking to small membrane fragments called apoptotic bodies.
cell itself shrinks and breaks up into membraneenclosed fragments called ……….
apoptotic bodies
excutioners of apoptosis
caspases (proteases)
How does caspases activation happen in apoptosis
when apoptosis is triggered, initiator caspases are activated (8,9), thus activating effector caspases (3,6,7), carry the responsibility of cell destruvtion
effector caspases are activated via
proteolytic cleavage
caspases
a family of proteases that cleave specific proteins in the cell
caspases activation is …………
irreversible switch for cell death
types of apoptosis
intrinsic apoptosis signaling
extrinsic apoptosis signaling
Intrensic apoptosis
signals are initiated from inside the cell
triggered by DNA damage, cellular stress or oncogene activation
BCL-2 family pathway (act at mitochondria)
mitochondria dependant
Mitochondria induce apoptosis when pro-apoptotic factors of the bcl2 factor outnumber anti--apoptotic factors (mitochondria storage site for apoptosis-regulating molecules) apoptosis-regulating
steps of intrinsic apoptosis
Step 1: Increase in the balance of proapoptotic (Bax/Bak of Bcl2 family) to antiapoptotic factors
Step 2: Release of cytochrome C, formation of apoptosome
Step 3: Activation of caspase 9 → activation of effector caspase 3 → apoptosis
……………. is a particularly dangerous form of cell stress
DNA damage
cells with damaged genomes may have suffered …………… that can lead to the development of ………….
mutations, cancer
DNA damage activates ………………, which ………….
ATM and Chk2 protein Kinases, phosphorylate p53
intrinsic apoptosis, DNA damage
➢ DNA damage activates the ATM and Chk2 protein kinases, which phosphorylate p53
➢ Accumulation of activated p53 leads to transcriptional activation of genes encoding the proapoptotic regulatory proteins
➢ This leads to activation of PUMA, release of cytochrome c from mitochondria, and activation of caspase-9 → activation of caspase 3→ apoptosis
Mutations in the …….. and ………genes are common in cancer which contribute to the ………..
P53, BCL2, Cell death resistance
PI3K/AKT
major regulator of cell survival and cell death in response to growth factor signaling by regulating both pro and antiapoptotic Bcl-2 family members
PI3K/AKT steps
➢ PI 3-kinase phosphorylates the membrane phospholipid PIP2 to PIP3
➢ PIP3 activates the serine/threonine kinase Akt
➢ One key substrate for Akt is the proapoptotic regulatory protein called Bad.
➢ Akt inhibits Bad→ inhibits apoptosis and promotes cell survival
➢ Bad is similarly a substrate for the Ras/Raf/MEK/ERK pathway
➢ Growth factor deprivation → activation of Bad → activates the intrinsic pathway of apoptosis.
Extrinsic apoptosis signaling
Initiated from outside the cell
from other cells from the extracellular matrix
Most well-known extrinsic pathways
Fas/fasl
tnf/tnfr
extrinsic pathway steps
➢ Step 1: Ligand (e.g. TNF) binds to cell death receptor (e.g. TNF receptor)
➢ Step 2: Activation of Caspase 8
➢ Step 3: caspase-8 can directly cleave and activate effector caspases (e.g. caspase 3)
➢ Step 4 (in some cases): caspase-8 cleaves the proapoptotic proteins of BCL-2, which activates the mitochondrial pathway of apoptosis, leading to caspase-9 activation → activate effector caspases (e.g. caspase 3)
Apoptosis induced by activation of Fas is responsible for
killing target cells of the immune system, such as cancer cells or virus infected cells.
Response to caspases
Caspases bring about the events of apoptosis by cleaving more than 100 different target proteins, resulting in DNA fragmentation and cell condensation.
inhibitor of DNases: DNA fragmentation
Nuclear lamins: nucleus frag
Cytoskeletal: disruption, cell frag, membrane blebbing
golgi matrix: golgi frag
scramblase: translocation of phosphatidylserine to the cell surface
Apoptotic cells and cell fragments are efficiently recognized and ………….by both …………..and …………..
Phagocytosed, macrophages, neighboring cells
The removal of apoptotic cells is mediated by the expression of socalled ……. on cell surface
eat me signals
……………… is usually in the inner leaflet of plasma membrane, but translocates outside during apoptosis and recodnized by ………..
phosphatidylserine, phagocytic cells
T or F cells that die by apoptosis are rapidly removed from the tissue
True
How can apoptosis be detected?
Annexin V: Fluorescently labeled Annexin V protein can be used to detect PS that is exposed on the outside of apoptotic cells (by fluorescent microscope, plate reader, flow cytometry…etc)
caspases expression whether gene or protein
live cell imaging of caspase 3 with fluorscent dye
DNA fragmentation (tunnel assay)
Medication target for apoptosis
chemotherapy
radiptherapy
Bcl-2 inhibitors
immunosuppressives
chemotherapy for apoptosis
ex: doxorubicin
severe DNA double stranded breaks
ATM activation and the phosphorylation of p53 resulting in capsapse 9 activation and apoptosis in cancer cells
Radiotherapy
cause DNA damage
→ ATM activation → p53 phosphorylation → Caspase 9 activation → apoptosis
Bcl2 inhibitors
ventoclax, apoptosis in leukemia cells
immunosuppressive meds
for autoimmune disease ex: arthritits, rheumatoid, lupus
ex: anti-TNF drugs as infliximab
Autophagy
Cell eats its own content
remove unnecessary or dysfunctional components in a lysosome dependant regulated mechanism
Importance of autophagy
survival during nutrient deprivation: autophagy increased degradation of cellular proteins and other macromolecules, allowing their components to be degraded as a source of energy or reutilized for essential functions
quality control: remove defective organelles or proteins
pathogen removal/ defense: during infection by Toll-like receptors that recognize pathogenassociated molecular patterns (PAMP), allow autophagy to kill pathogens to the innate and acquired immunity
nutrient rich conditions
mTOR active = no autophagy
nutrient deprivation
mTOR inhibited= autophagy
t or f mTOR is a positive regulator of autophagy
false
…………….. is a common antitumor medication that inhibits mTOR
Rapamycin
mTOR full name
mamilian target of rapamycin
function of rapamycin
inhibitor of the protein kinase mTOR → promoting cell death via autophagy in cancer cells
Mechanism of autophagy
initiation: nutrient deprivation, stress, infection
autophagosome formation: nucleauation and elongation of a phagophore
fusion with lysosome: form autolysosome
degradation of cellular components: by enzymes in lysosome
autophagosome is formed via
nucleation and elongation of a phagophore (double membraned vesicle)
function of autophagosome
engulf cellular components, such as organelles, macromolecules, and bacteria.
What happens if autophagy is impaired
Neurodegenerative disease: es: parkinson’s. accumlation of damaged organelles or macromolecules in neurons
Cardiovascular disease: ex: atheroscelerosis, accumlation of damaged cells in the heart or blood vessels
Necrosis
passive due to injury or stress, infection, toxins
unregulated breakdown of cells and tissues
accompanied by inflammation
ex: ischemia/ lack of oxygen
necrosis happen in ……. conditions while apoptosis in ……….. or ……….
pathological, physiological, pathological
…….. is the body’s response to injury or infection
inflammation
main feautures of necrosis
Cell swelling
cell membrane disruption
cellular leakage
inflammatory responses
DAMPS activation
DAMPS
Damage-Associated Molecular Patterns are molecules released from dying cells as a result of necrosis.
summary
Necrosis vs apoptosis
Experimental work to answer the research question
•Detection of activated caspases, such as caspase-3 and caspase-7: qPCR, western blot
•Analysis of DNA fragmentation using techniques like the TUNEL assay : fluorscent microscope
•Measurement of phosphatidylserine externalization using Annexin V staining