Energy Science
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
Derivation of internal energy being independent of volume

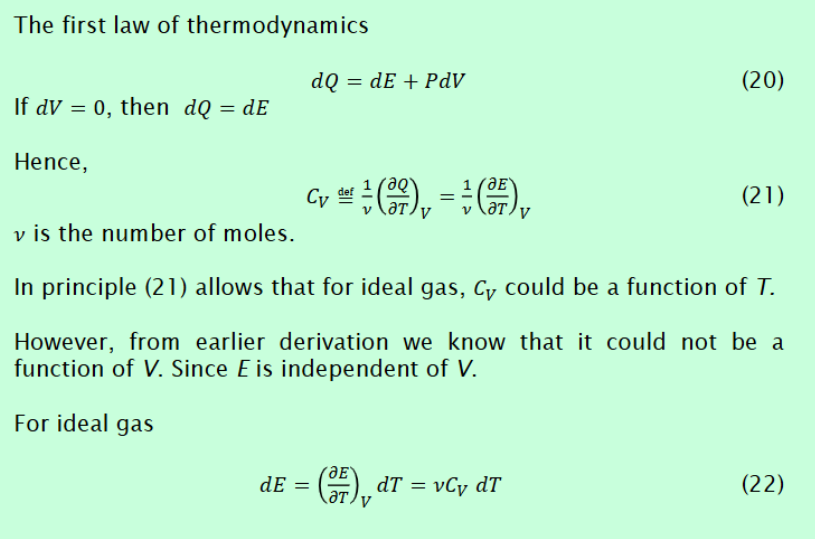
Derive C_v constant volume specific heat for regular and ideal
Derive C_p (ideal)

Derive adiabatic, ideal gas system constant values

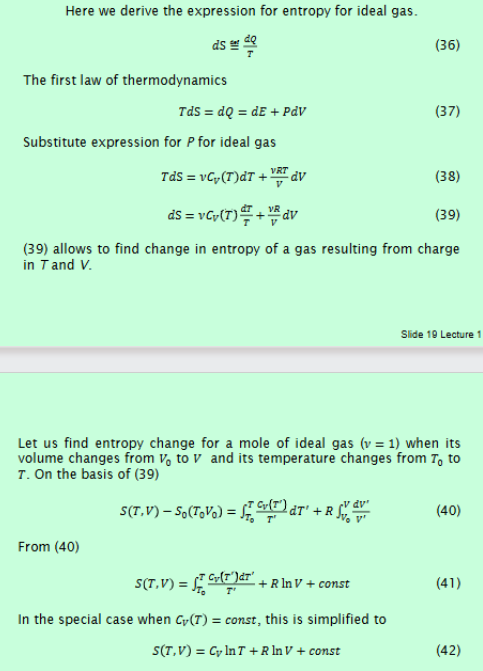
Derive entropy for ideal gas
Describe the 4 laws of thermodynamics and give overall expression
Oth if 2 systems are in thermodynamic equilibrium with a third system, they are at equilibrium with e.o.
1st delta E = - delta W + delta Q
2nd thermally isolated system changing macrostate, entropy tends to increase
Not isolated, quasi static infinitesimal change gives delta S = delta Q / T
3rd at T tends to 0 K, S tends to a value S_0
Derive first general relation (maxwell)

Derive second general relation (maxwell)

Derive third general relation (maxwell)

Derive fourth general relation (maxwell)

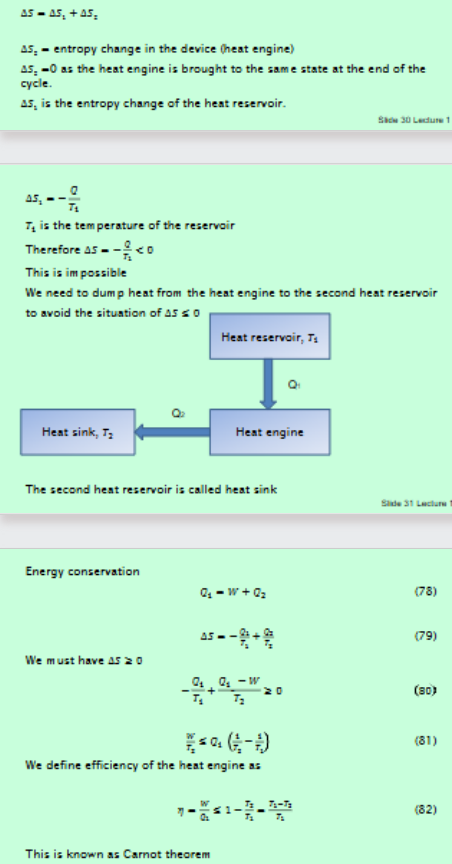
Derive Carnot theorem
Name 3 useful formulae for ideal gases
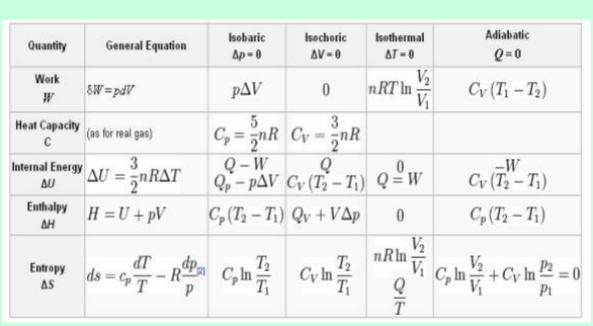
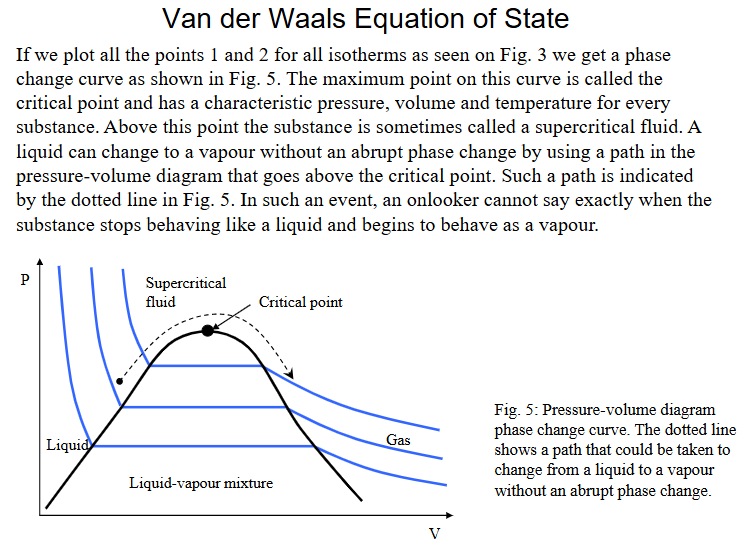
Van der Waals equation of state and explain extra terms
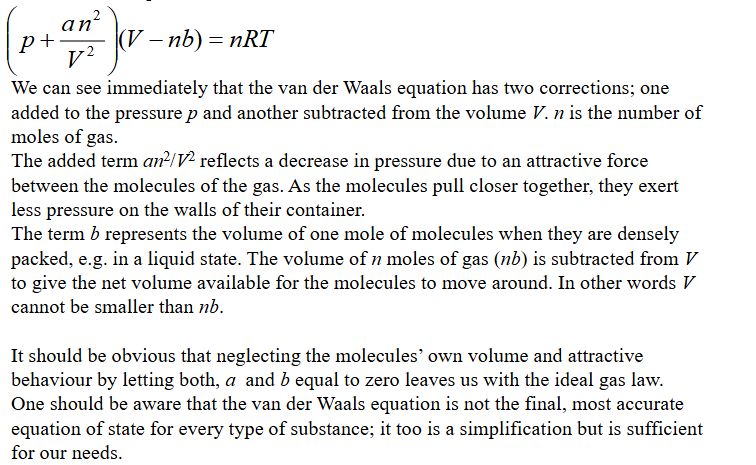
Problem with the PV graph produced by van der waals equation of state
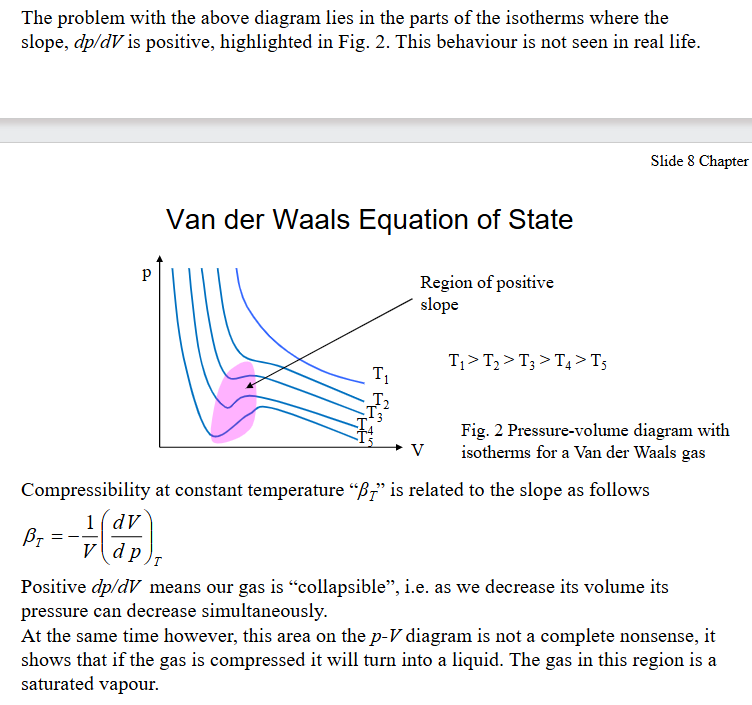
Explain how the problem with VdW eq. of state PV diagram was ‘fixed’ or explained
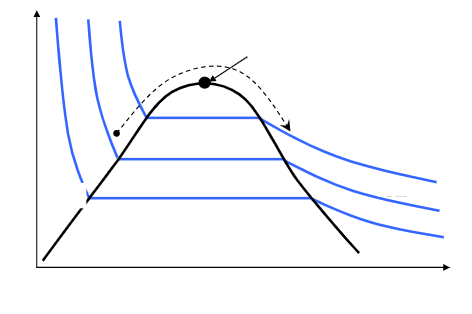
add the 5 labels and axis with a brief description of the graph
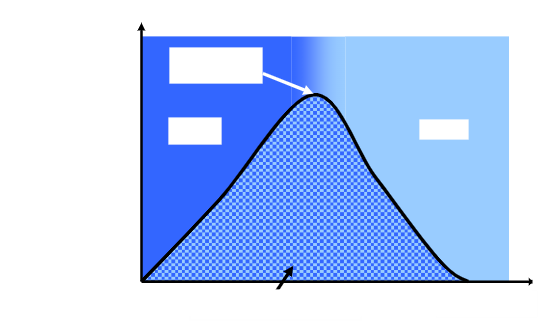
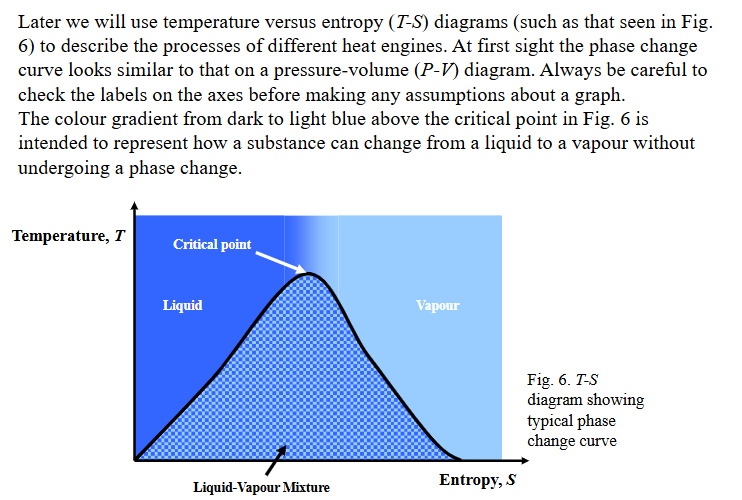
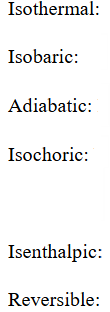

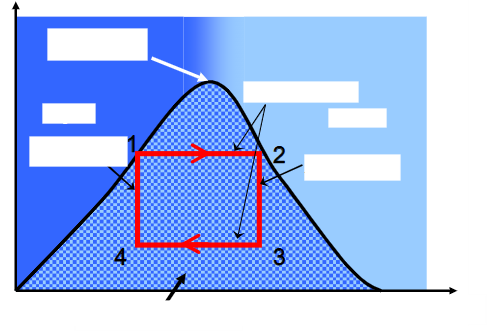
name the cycle, what its used in and fill in the covered terms.
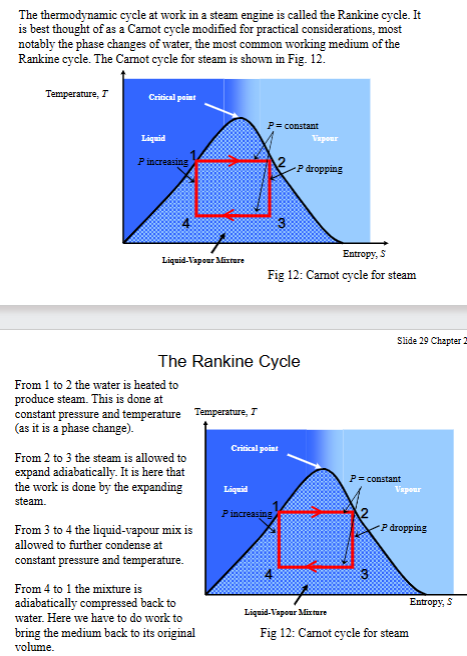
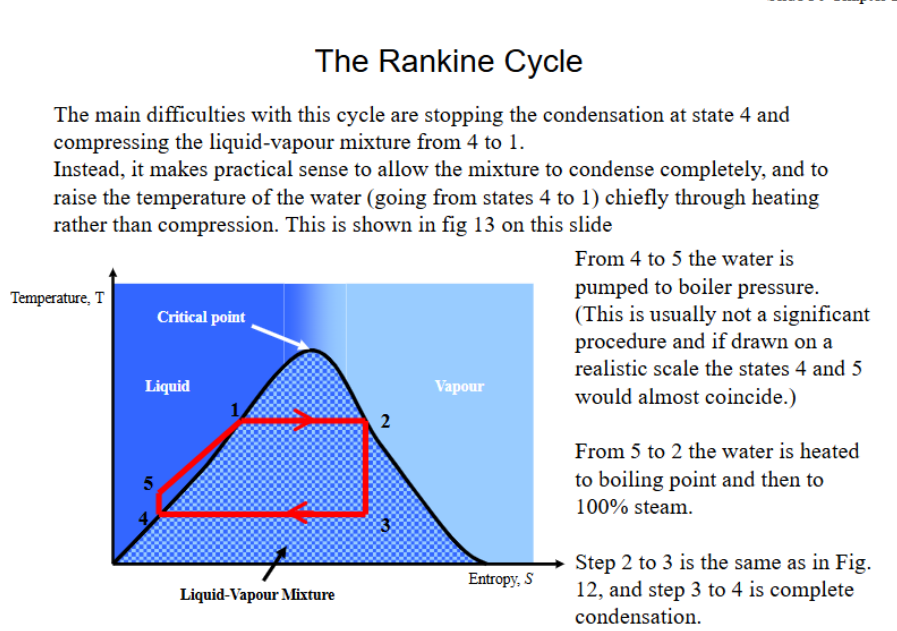
What is the Rankine cycle, draw and describe.
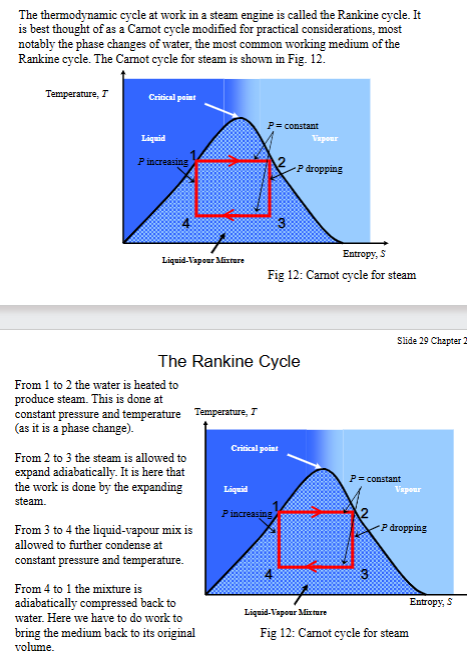
How is the Rankine cycle adjusted to improve its practicality
Ideal and actual efficiency

Work ratio, definition and explanation

significance of the work ratio
when the W_in and W_out are reduced from their ideal values then the work ratio shows how significant the reduction in efficiency of the system will be, work ratio near 1, small difference, small work ratio, big difference.
working medium, air vs water