1.3.1b - Consolidate: Limiting Reagent & Mole Triangles
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Last updated 8:06 PM on 5/17/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
1
New cards
Limiting Reagent:
In most chemical reactions, there are…
In most chemical reactions, there are…
two or more reactants
* one has always been in excess
→ More of it than needed; does not cause the reaction to stop, as it is not completely used up (some left over unreacted)
* one has always been in excess
→ More of it than needed; does not cause the reaction to stop, as it is not completely used up (some left over unreacted)
2
New cards
Limiting Reagent:
The other reactant that determines (or limits) how much product is formed
3
New cards
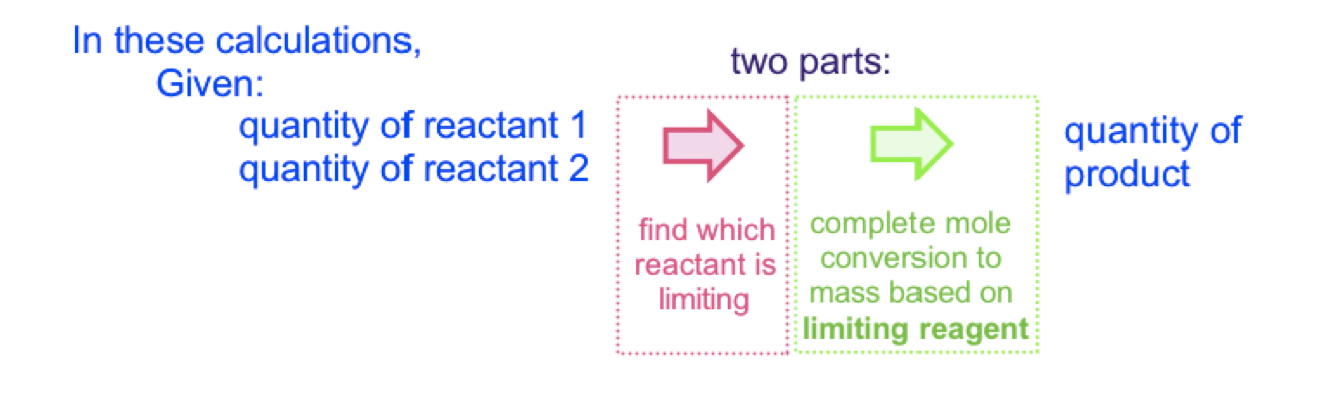
In these calculations:
\* Image Inserted
4
New cards

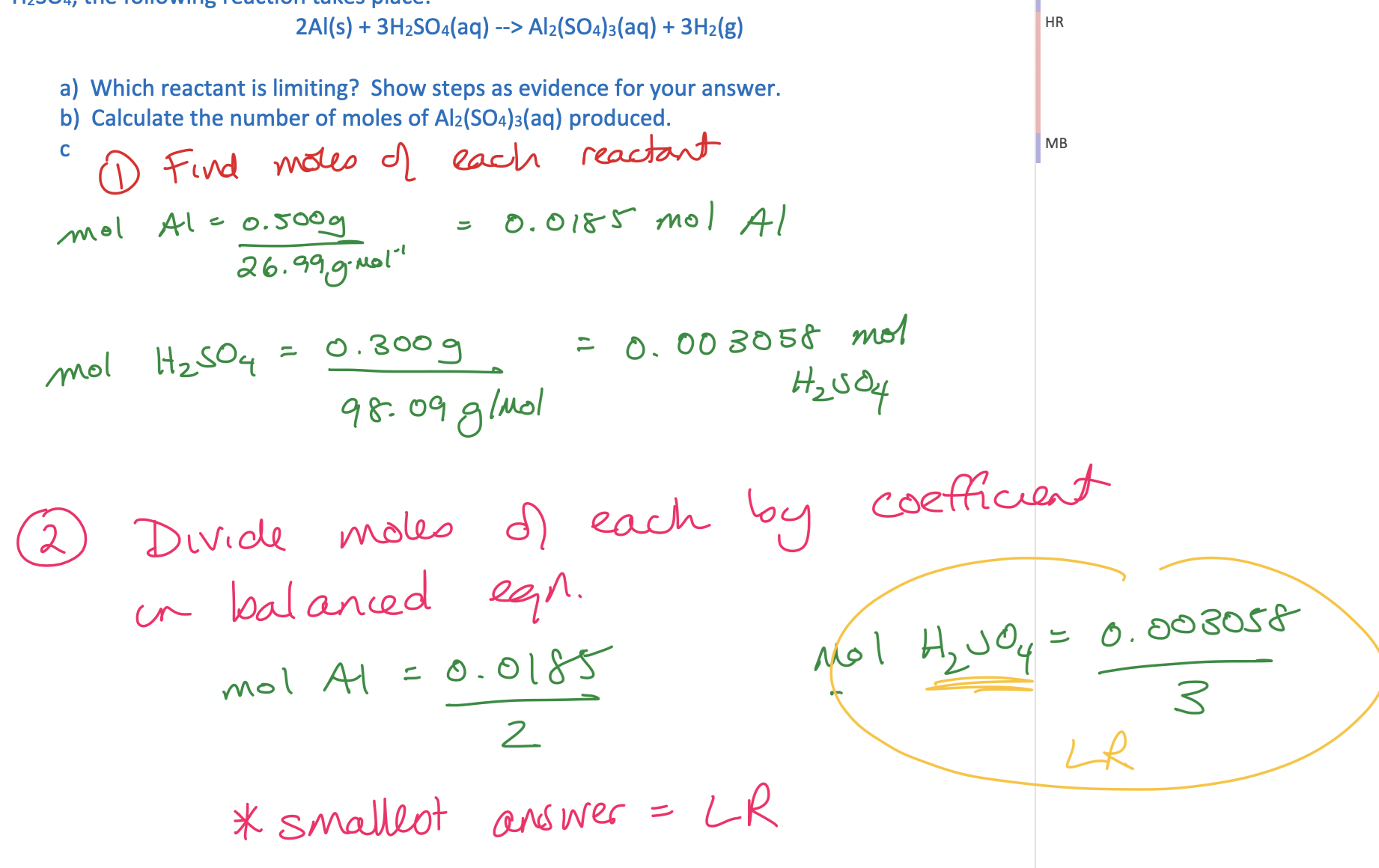
Practice: \*Image Inserted
Answer \*Image Inserted
(continue additional practices in OneNote note)
(continue additional practices in OneNote note)
5
New cards