Lab 4 Melting Point Analysis
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Purpose:
To purify an impure compound and verify its identity (acetanilid or phenacetin) by melting point analysis
Recrystallization
a purification technique for impure solids that involves dissolving the solid in a minimal amount of hot solvent, then slowly cooling the solution to allow the pure compound to crystallize while impurities remain dissolved
Phenacetin Structure
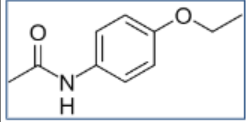
Acetanilide Structure
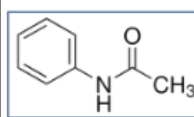
Chemicals used
Phenacetin and Acetanilide
Melting point (MP)
The temperature where the solid and liquid phases of a substance are in equilibrium at a pressure of 1 atm
Melting point analysis is used
To check the purity of a compounds or to identify unknown compounds
When pure substances melt:
The solid and liquid phases of that substances are at equilibrium
When a substance is impure melts:
Its melting point broadens and is lowered slightly (freezing point depression)
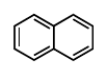
Naphthalene structure
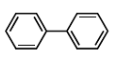
Biphenyl
Mixed melting point
technique which is used to determine the identity of unknown compounds
Items needed for this experiment:
Melting point apparatus aka Mel-temp, thermometer, and three melting point capillaries
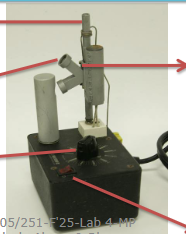
Melt-temp Apparatus components
Thermometer port, eye port, dial control, capillary tube port, and on/off switch
A pure substance has
a single melting point
Good recrystallization solvent
High solubility in hot solvent and low solubility in cold solvents
Results
Unknown A is Phenacetin