UNIT 1: Atomic Theory - Democritus to Bohr-Rutherford
1/23
Earn XP
Description and Tags
SCH3U1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Democritus’ contribution to the atomic model
Studied the existence and behaviour of the indivisible particle - make up everything
Kanada’s contribution to the atomic model
Atoms are the smallest state of matter, either moving or not moving.
Dalton’s contribution to atomic model
Atoms of different elements can be distinguished based on their varying atomic weights.
—
Matter is comprised of individual, indestructible atoms with distinct masses and properties.
—
Atoms cannot be created/destroyed
Atoms of the same material are the same, unique to different elements
Atoms can’t change from one to another
Compound elements are formed when different atoms join together
Dalton’s law of Partial Pressure
As long as two elements don’t react with each other, the pressure exerted by two gasses is the sum of the pressure of each gas individually.
Dalton’s atomic model
Each atom is a big ball

Thomson’s atomic model
Atoms are not indivisible balls with nothing inside of them, but they have negative subatomic particles called Electrons, while the atom itself is positive
Thomson’s experiment
In a cathode ray, positive and negative magnets were placed on each side. When an element was put into the tube and turned on, it consistently went to the positive side, but the element normally isn’t negatively charged.
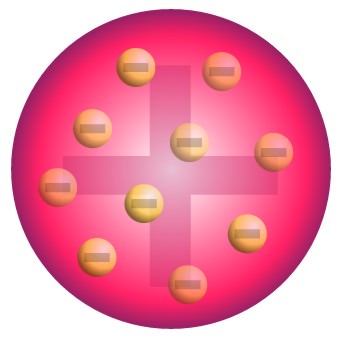
Thomson’s atomic model
Plum pudding
Rutherford’s contribution to the atomic model
Created the idea of a distinct idea of protons in the centre (the nucleus), and that electrons orbit the nucleus, in an unorganized manner.
Rutherford’s experiment
Gold Foil experiment: Alpha particles were shot at a piece of gold. Most went through, however some were reflected in a different manner, only possible if there was a nucleus in the atom, and lots of empty space.
Rutherford’s model
Electrons orbit the nucleus of protons, eventually falling into it.

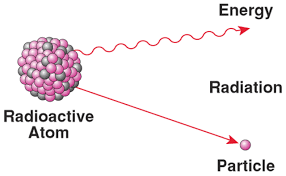
Becquerel & Curie’s contribution to the atomic model
Discovered radioactivity, which is the emission of radiation caused by the decay of nuclei in certain elements and isotopes.
Radiation can be deflected by a magnetic field, therefore consists of charged particles.
Becquerel & Curie’s experiment
Placed a radioactive material on wrapped in paper on a photographic plate wrapped on paper. The radiation from the salt transferred to the photographic plate, making it appear that it was exposed to light.
Becquerel & Curie’s model of the atom
Radiation
Chadwick’s contribution to the atomic model
Discovered the neutron
Chadwick’s experiment
Protons should account for almost all the mass of the atom, but it only accounted for half. Bombarded beryllium with alpha particles and when struct, emitted neutral rays. The new particle had no charge but almost the same mass as a proton.C
Chadwick’s model of the atom
All subatomic particles are present, improper electron configuration
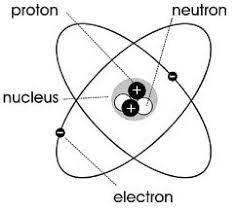
Bohr’s model contribution to the atomic model
Discovered that electrons are in organized orbits, creating the most accurate representation of the atom
Bohr’s experiment
If electrons orbit and fall into the nucleus, all colours of light should be emitted. This was not the case. Discovered that certain elements will emit certain wavelengths of light when electrons gain/loose energy (going up and down orbitals)
Bohr’s model of the atom
Value of A in atomic notation
Atomic Mass
Value of Z in atomic notation
Number of protons
Value of X in atomic notation
Elemental symbol
Dalton's 5 Principles
atoms couldn't be created or destroyed.
All atoms of the same element are identical; different elements have different atoms.
A given compound always consists of the same kinds of atoms in the same proportions.
The atoms of one element can not be converted into the atoms of any other element
“Compound elements” (compounds) are formed when different atoms join together to form “compound atoms” (molecules).