Solutions
0.0(0)
Card Sorting
1/35
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
1
New cards
disassociation
separation of ions from each other
2
New cards
solvation
surrounding of solute particles by solvent
3
New cards
hydration
solvation by H2O
4
New cards
insoluble
stronger attractive forces w/in crystal and can’t be separated
5
New cards
solids with low MP and low Hf
soluble
6
New cards
miscibility
mutual solubility of **2 LIQUIDS**
7
New cards
complete miscibility
soluble in all proportions
8
New cards
immiscible
won’t dissolve at all
9
New cards
alloy
solid-solid solution
10
New cards
solution equilibrium
number of particles in soln = number of particles leaving; **OCCURS AT SATURATION**
11
New cards
Temperature
what solubility depends on
12
New cards
solubility
amt. of solute that will dissolve
13
New cards
1. Increase in T
2. Stirring
3. Increasing Surface Area (grinding)
factors that affect solution rate
14
New cards
hot
solid to liquids, more soluble in ___ H2O
15
New cards
cold
gases are more soluble in ___ H2O
16
New cards
Molarity (M)
moles solute/ liters of solution
17
New cards
Henry’s Law
the more pressure a gas exerts on a liquid, the more of that gas will dissolve in the liquid
18
New cards
initial concentration/initial partial P
final concentration/ final partial P
19
New cards
concentrated
lots of solute for the amount of solvent
20
New cards
>3M
concentrated
21
New cards
dilute
22
New cards
dilute
little solution for amt. of solvent
23
New cards
molality (m)
moles solute/ kg solvent
24
New cards
mole fraction (X)
moles solute/ total moles solution
25
New cards
M₁V₁ = M₂V₂
dilution equation
26
New cards
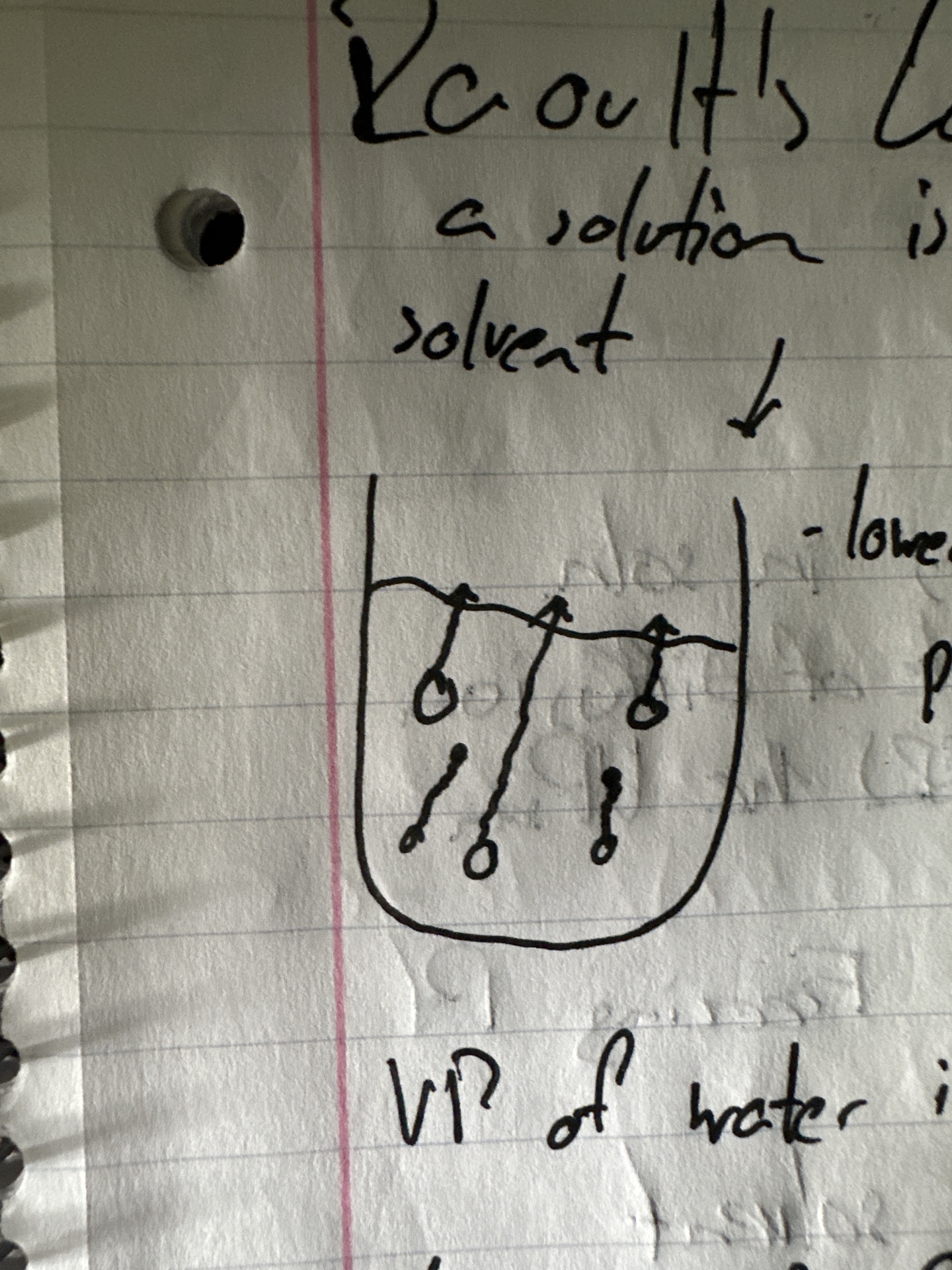
Raoult’s Law
the vapor pressure of a solution is lower than that of a pure solvent
27
New cards
P (solution) = X (mole fraction of solvent) x original VP (solvent)
Raoul’s Law equation
28
New cards
VP (lowering) = X (mole fraction of solute) x P (original pressure of solvent)
VP lowering equation
29
New cards
1. depends on # of particles on soln
2. includes conductivity, VP, BP, MP, rate of diffusion
3. adding solute to a solvent **LOWERS** the VP
Colligative Properties
30
New cards
lowers Freezing Pt
creating a solution _____ the freezing pt.
31
New cards
increases BP
creating a solution _____ the BP
32
New cards
.512ºC/molal
molal BP elevation for H₂O
33
New cards
1\.86ºC/molal
molal FP elevation for H₂O
34
New cards
∆T = K(f or b) x molality x number of particles
∆T equation
35
New cards
1. Find Molality
2. Determine ∆T
3. + or - the ∆T from the original BP or FP
steps to find new fp or bp
36
New cards
grams/mole
molecular mass determination