The nature of substances and chemical reactions
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
Q: What is an element?
A: A substance made of only one type of atom.
Q: Can elements be broken down into simpler substances?
A: No, elements cannot be broken down into a simpler substance.
Q: What happens when iron is heated?
A: It produces molten iron.
Q: What are elements the building blocks of?
A: All other substances, such as compounds.
Q: How are atoms arranged in solid elements?
A: They are tightly packed together.
Atoms in gases often go round in pairs, what is a molecule with two atoms in it called?
A diatomic molecule
Q: What is a compound?
A: A substance made of two or more different types of atom that are chemically bonded.
Q: How is carbon dioxide formed?
A: One carbon atom reacts with two oxygen atoms in a chemical reaction.
Q: Is it easy to separate elements in a compound?
A: No, it is very difficult to separate them.
Q: How are the properties of a compound compared to the original elements?
A: They are often totally different.
Q: Does iron sulfide behave like iron or sulfur?
A: No, it’s not magnetic like iron or yellow like sulfur.
Q: How are mixtures different from compounds?
A: There is no chemical bond between the parts of a mixture.
Q: How can mixtures be separated?
A: By physical methods like distillation.
Q: What are the properties of a mixture like?
A: A combination of the properties of its separate parts.
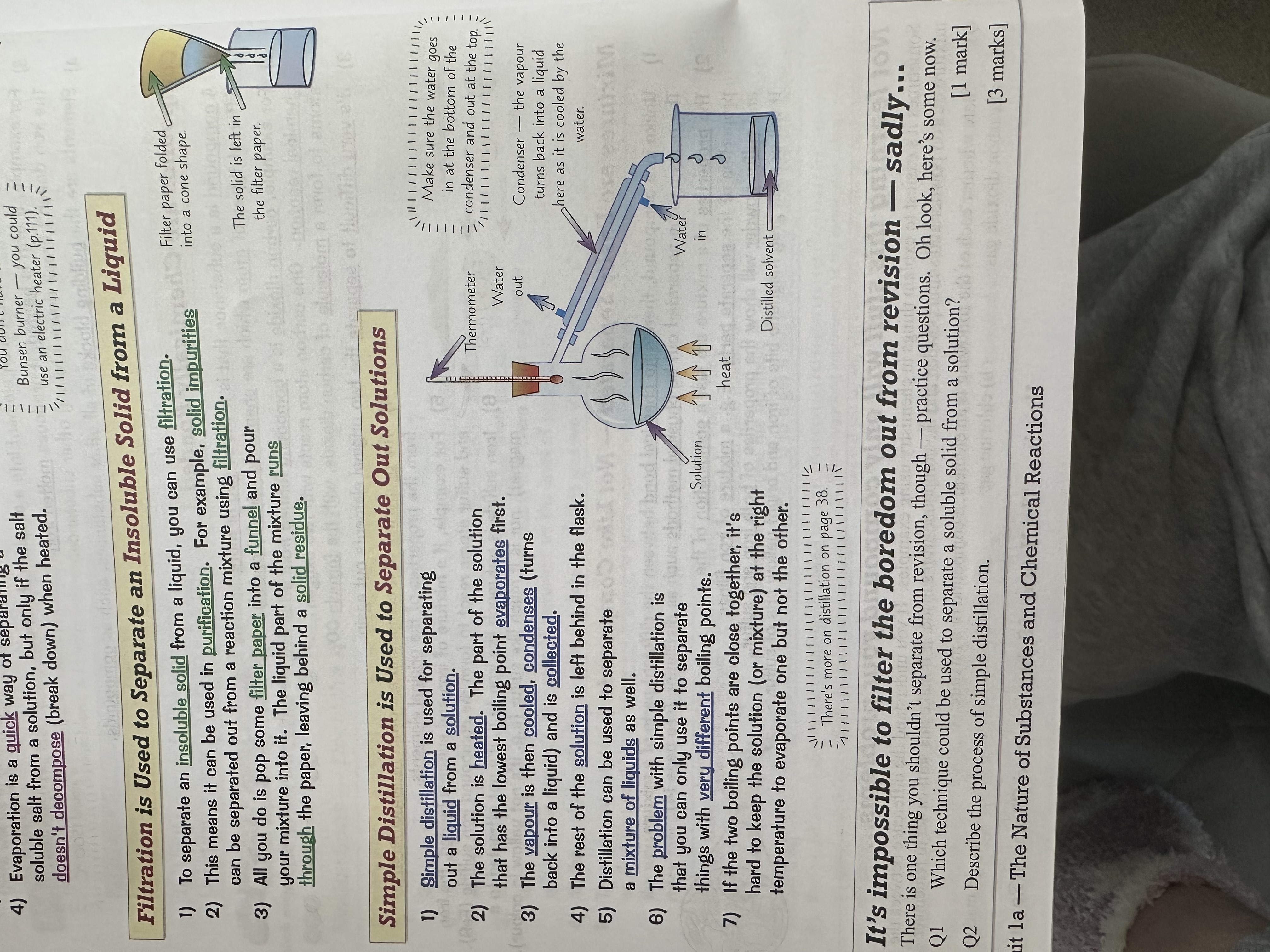
How do we use evaporation to separate soluble solids from solutions.

How do we use simple distillation to separate out solutions?
Q: Why must the solvent be placed below the baseline?
A: To prevent the ink spots from dissolving directly into the solvent at the start.
Q: What carries the dyes up the paper in chromatography?
A: The solvent, which seeps up the paper, carrying the inks with it.
Q: Why do different dyes move different distances in chromatography?
A: some dyes will be more attracted to the paper so spend more time stuck to it and not travel as far, whilst others will be more soluble in the solvent so will travel further up the paper
Q: What does an Rf value tell you in chromatography?
A: An Rf value is the ratio between the distance travelled by the substance and the distance travelled by the solvent
Q: What are ionic compounds made of?
A: A positively charged part and a negatively charged part.
Q: What is the overall charge of any ionic compound?
A: Zero — all the positive and negative charges are balanced
Q: How do you work out the formula for an ionic compound?
A: You can use the charges on the individual ions present to work out the formula for the ionic compound

Q: What do chemical symbols represent?
A: Atoms of each element can be represented by a one or two letter symbol.
Q: What does a molecular formula show?
A: The number of atoms and each type of atom in a molecule.
Q: What is a structural formula?
A: A structural formula shows the atoms and covalent bonds in a molecule as a picture.

Q: What do brackets in a formula indicate, like in CH₃(CH₂)₂CH₃?
A: They show repeated groups; (CH₂)₂ means there are 2 CH₂ units.
Q: What is the molecular formula of CH₃(CH₂)₂CH₃?
A: C₄H₁₀ (4 carbon atoms, 10 hydrogen atoms).
Q: What are the reactants and products in the reaction of magnesium with oxygen?
A: Reactants: magnesium and oxygen; Product: magnesium oxide
Q: What is the word equation for the reaction between magnesium and oxygen?
A: magnesium + oxygen → magnesium oxide
How do we balance an equation

A:
Calculate Mᵣ of CO₂ = 44
Number of moles = 66 ÷ 44 = 1.5 moles
A:
Mass = No. of moles × Mᵣ
Mᵣ = Mass ÷ No. of moles
No. of moles = Mass ÷ Mᵣ
A:There are 4 moles of carbon in 4 moles of CO₂.
Mass = 4 × 12 = 48 g
Q: How many moles are in 60 g of magnesium? (Ar of Mg =24)
Q: What is the mass of 2.5 moles of MgO? (Mr of MgO = 40)