orgo chapter 19 rxns
1/32
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
benzene +Br2 + FeBr3
bromobenzene + HBr
benzene + Cl2 + FeCl3
chlorobenzene + HCl
Nitration
benzene + HNO3 + H2SO4
Benzene-NO2 + H2O
sulfonation
benzene + H2SO4 + heat
benzene-SO3H
fridel crafts acylation
benzene + acyl chloride + AlCl3/H2O
benzene- in ketone
does not happen under meta directors
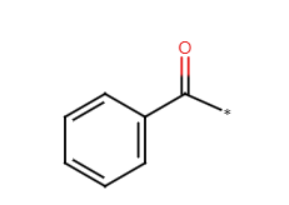
fridel crafts alkylation
benzene + RCl + AlCl3
benzene w alkyl chain
does not occur for meta directors
gatterman koch
CO + HCl + Benzene + high pressure + AlCl3/CuCl
benzaldehyde
benzene-carbonyl + H2/PdC
benzene-CH2R (methylene)
benzene-carbonyl + H2NNH2, HO-, heat (wolff-kishner)
benzene-CHR (methylene)
bromobenzene + R-B-(OR)2 + PdL2 + HO- (suzuki coupling)
benzene-R
bromobenzene + gilman
benzene-R
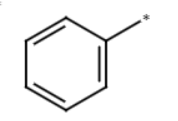
methylbenzene + Br2/hv
bromomethylbenzene
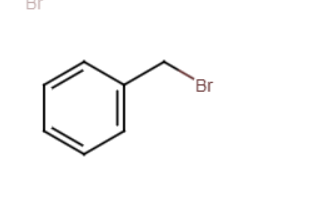
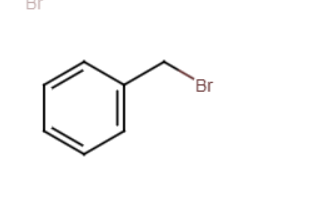
bromomethylbenzene + Z-
zmethylbenzene (replaces bromine with nucelophile)
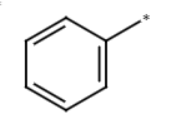
methylbenzene + H2CrO4
benzene-COOH
benzene-NO2 + H2/PdC
benzene-NH2
benzene-NH2
will not undergo fridel crafts, must be protected by acetylation to be nitrated
benzene-NH2 + NaNO2 + HCl @ 0 deg
azide
benzene-N+≡N
azide + CuBr
bromobenzene + N2(g)
azide + CuCl
chlorobenzene + N2(g)
azide + CuC≡N
benzene-nitrile + N2(g)
azide + HBF4 + Heat
fluorobenzene + N2(g) + BF3 +HCl
azide + KI
iodobenzene + N2(g) + KCl
azide + H3O + heat
phenol + N2(g) + KCl
azide + Cu2O + Cu(NO3)2 + H2O
phenol + N2(g)
azide + H3PO4
benzene (elimination of azide) + N2(g)
benzyne rxn
NaOH, H2O, 340deg, 170 atm
-Cl is leaving group, replaced by OH
OH attacks nearby H, which kicks off lg and forms 3 bond, then reacted w water
—NH2, —OH, —OR
strong activating, ortho para
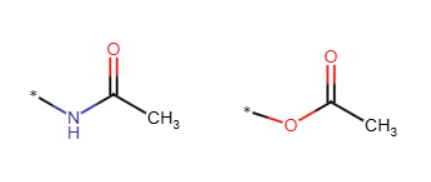
—amide, —ester (must be attached to noncarbon)
moderately activating, ortho para
—benzene, —R, —CH=CHR
weakly activating, ortho para
—halogen
weaking deactivating, ortho para
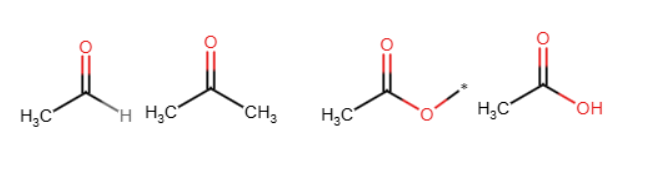
—aldehyde, ester, ketone, carb acid
moderately deactivating, meta
—NO2, —CN, —SO3H, —N+R3
strongly deactivating, meta
bromobenzene or chlorobenzene + -OH + 130 deg
nucleophilic aromatic substiution (NAS)
phenol