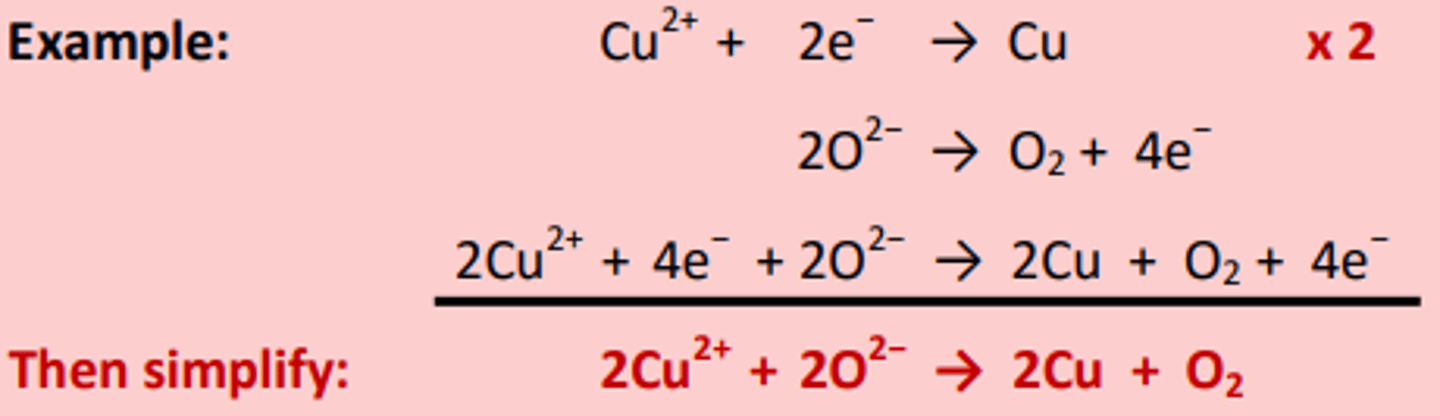3.1.7 Oxidation, Reduction and Redox Equations
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
O
I
L
R
I
G
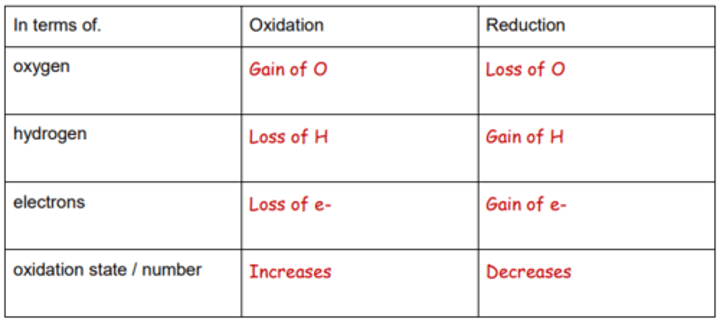
Oxidation
Is
Loss
Reduction
Is
Gain
Oxidation
Oxidation is the loss of electrons
Reduction
Reduction is the gain of electrons
Definitions:
Oxidation Numbers
The oxidation number of an atom is the charge that would exist on an individual atom if the bonding were completely ionic.
Elements in their standard states:
The oxidation number of each atom is zero. e.g. Cl2, S, Na and O2 all atoms have an oxidation number of zero.
In simple ions:
The oxidation number of the atom is the charge on the ion:
e.g. Na+ oxidation number = +1,
Mg2+ oxidation number = +2,
Br- oxidation number = −1
O2- oxidation number = −2.
Transition metals
The oxidation states are variable.
In other compounds and complex ions:
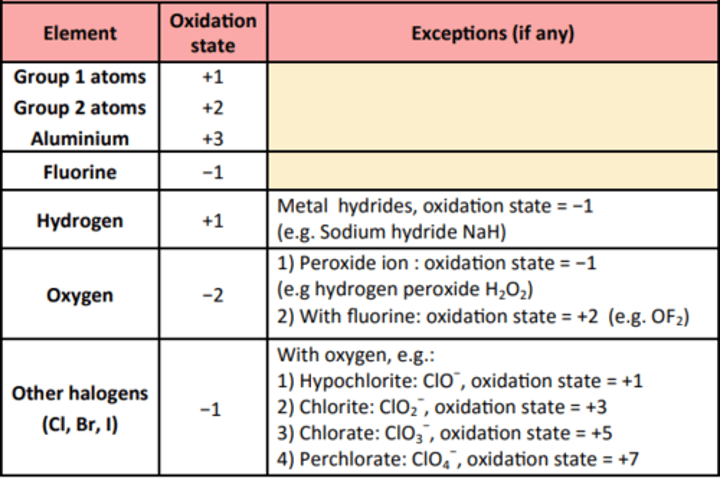
In compounds the sum of the oxidation states of the atoms is zero.
Example: SO3
Oxidation state of each O atom = −2
Sum of oxidation states of 3 x O atoms = −2 x 3 = −6
Therefore, oxidation state of S = +6 [+6 + 3x(−2) = 0 ]
In complex ions, the sum of the oxidation numbers on the atoms is equal to the overall charge on the ion.
Example: SO42-
Oxidation state of each O atom = −2
Sum of oxidation states of 4 x O atoms = −2 x 4 = −8
Therefore, oxidation state of S = +6 [+6 + 4(−2) = −2 ]
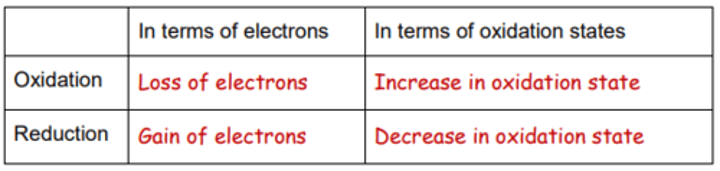
Using oxidation states to identify oxidation and reduction
During oxidation and reduction, the oxidation numbers of atoms change.
If an atom is oxidised, its oxidation state increases (it becomes more +ve or less -ve)
If an atom is reduced, its oxidation state decreases (it becomes less +ve or more-ve)
Using oxidation states in chemical nomenclature
For elements which can have more than one positive oxidation state then the oxidation state of that element is included in the name of the species
e.g. Copper(II) sulfate, copper has an oxidation state +2
In the nitrate(V) ion, nitrogen has an oxidation state of +5
Agents

An oxidising agent
Accepts electrons/ Electron acceptor
It brings about oxidation and is itself reduced.
A reducing agent
Donates electrons/ Electron donor
It brings about reduction and is itself oxidised.
Example of agents
The zinc has lost (donated) electrons (oxidation state has increased); it has been oxidised. Zinc is therefore the reducing agent.
The copper has gained (accepted) electrons (oxidation state has decreased); it has been reduced. Copper is therefore the oxidising agent.
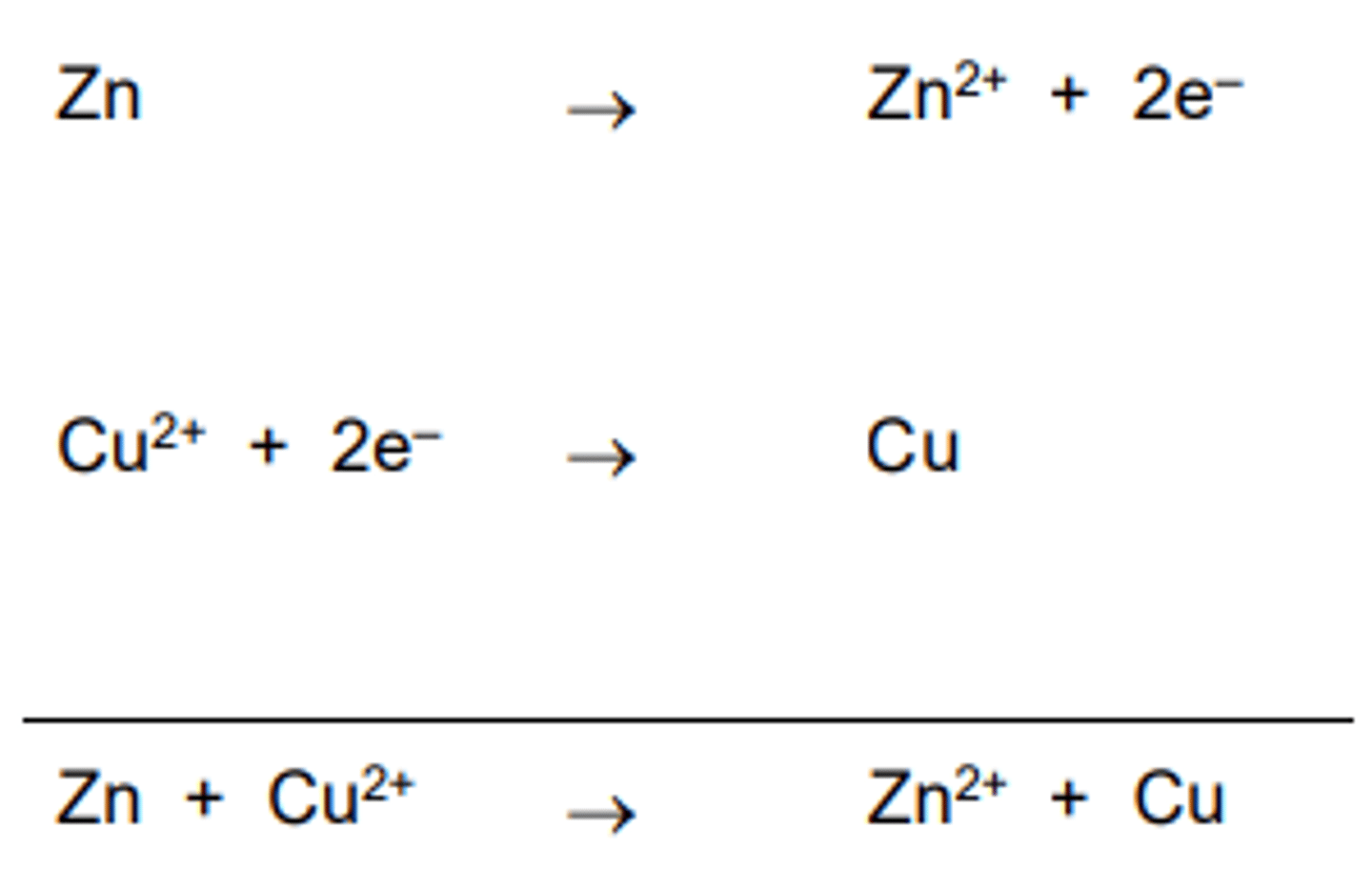
RA and OA- Example
Disproportionation
Disproportionation occurs when an element is simultaneously oxidised and reduced.
Disproportionation reactions are special examples of redox reactions.
e.g. Cl2 (g) + H2O (l) → HClO + HCl
Oxidation states of 0 +1 -1
Cl atoms
For Cl2 → HClO the oxidation state of the Cl atoms increases from 0 to +1; these Cl atoms have been oxidised.
For Cl2 → HCl the oxidation state of the Cl atoms decreases from 0 to -1; these Cl atoms have been reduced.
Half Equations
Half equations clearly show which element has been oxidised and which element has been reduced in a reaction.
If the electrons are on the product (right) side =
Oxidation process
If the electrons are on the reactant (left) side =
Reduction process
Oxidation: Mg → Mg 2+ + 2e−
The Mg oxidation number: 0 to +2
Reduction: Cu2+ + 2e− → Cu
The Cu oxidation number: +2 to 0
Writing Half Equations
Write down the two species involved on either side of the reaction arrow.
Then:
1) Balance the numbers of the element which is oxidised or reduced.
2) Then balance any other elements apart from O and H. E
3) Balance O atoms by adding water. O
4) Balance H atoms by adding + H. H
5) Add the required number of electrons to C make the charge equal on both sides of the equation
Overall redox equations
Half equations consider either a reduction or oxidation process, in reality, you cannot have oxidation without reduction and vice versa.
Oxidation and reduction always occur simultaneously.
Any reaction which involves oxidation of one species and reduction of another is called a redox reaction.
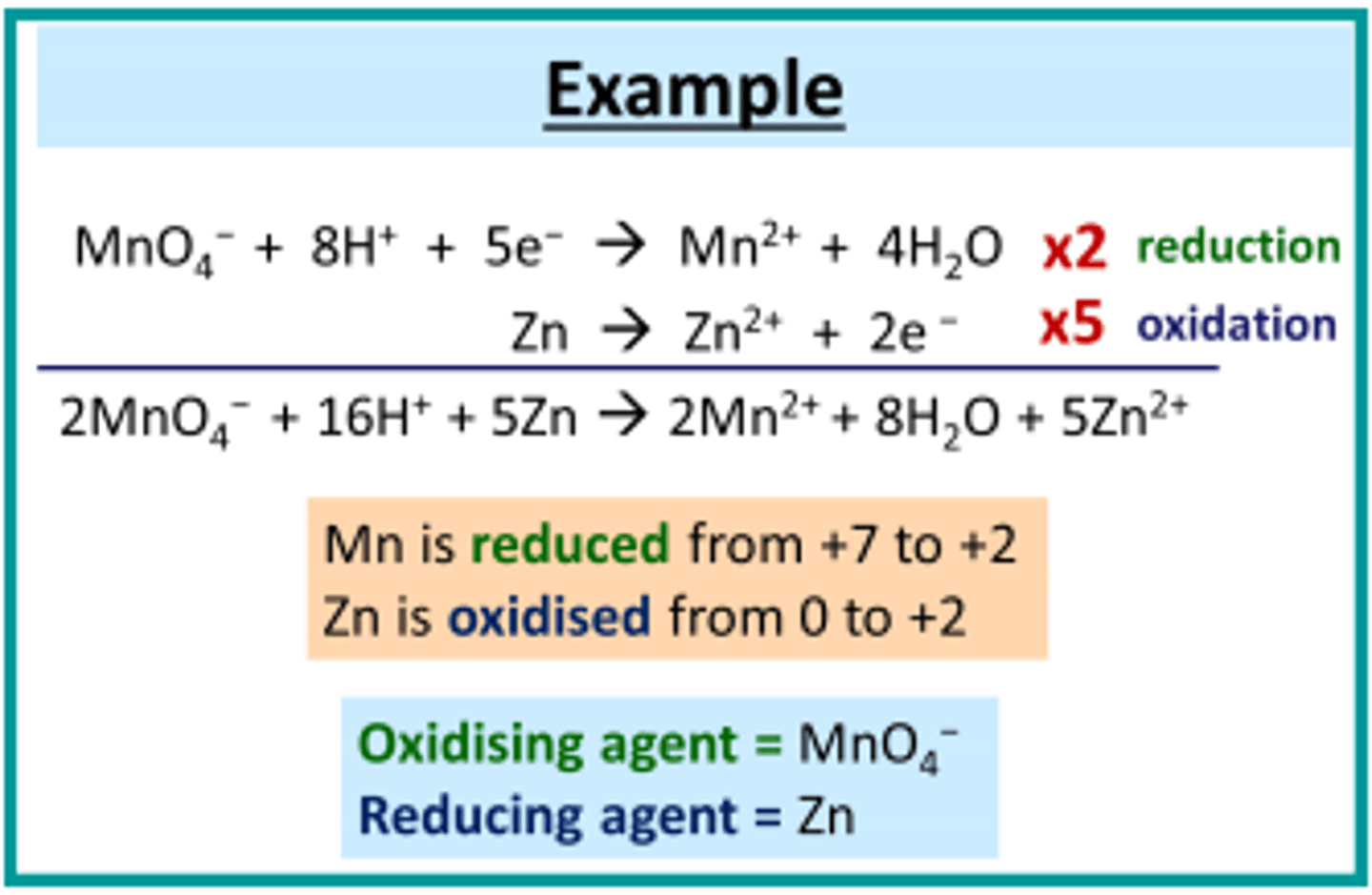
Combining half equations for redox reactions
A balanced redox equation comes from combining an oxidation half-equation with a reduction half-equation in such a way that the total number of electrons gained is equal to the total number of electrons lost.
If the number of electrons in each half equation does not match then you must multiply one or both equations to even out the electrons.
Remember you can have half numbers of diatomics like O2, N2 Cl2.
Combining Half Equations- Example:
The electrons cancel on both sides of the equation.