Water, Electrolyte, and Acid-Base Balance: Key Concepts and Regulation
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
What is the balance concept in relation to water and electrolytes?
the need for intake of water and electrolytes to equal their output to maintain homeostasis.
What percentage of body weight is composed of water in males and females?
Males: 63% water; Females: 52% water.
What are the two main compartments for body fluids?
Intracellular Fluid Compartment (ICF) and Extracellular Fluid Compartment (ECF).
What is the primary fluid in the Intracellular Fluid Compartment?
Fluid within cells, comprising about 63% of total body water.
What is the primary fluid in the Extracellular Fluid Compartment?
Fluid outside of cells, including interstitial fluid, plasma, and lymph, comprising about 37% of total body water.
What regulates the movement of fluids between compartments?
Hydrostatic pressure drives fluid out of plasma, while osmotic pressure causes fluid to return to plasma.
How does sodium ion concentration affect fluid movement?
Sodium ion concentrations are crucial because water follows sodium through cell membranes.
What are the main routes by which water enters the body?
consumption of liquids, moist foods, and dehydration synthesis.
What triggers the thirst response when the body loses water?
An increase in osmotic pressure of extracellular fluid (ECF) stimulates osmoreceptors in the hypothalamus.
What is the role of antidiuretic hormone (ADH) in water regulation?
increases water reabsorption in the kidneys, reducing urine output when ECF is concentrated.
What happens to ADH release when there is excess water in the body?
ADH release decreases, leading to reduced water reabsorption and increased urine output.
What is electrolyte balance?
when the intake of electrolytes equals their output.
How do electrolytes primarily enter the body?
obtained from food, beverages, and as byproducts of metabolic reactions.
What is the main mechanism for electrolyte output?
through kidney function and urine production.
What physiological systems help return blood pH toward normal during acid-base imbalances?
Both the renal and respiratory systems
What does pH number indicate about body fluids?
The pH number describes the acidity or alkalinity of a body fluid.
What are the major sources of hydrogen ions in the body?
Major sources include metabolic processes, such as the breakdown of glucose and fatty acids.
What distinguishes strong acids from weak acids?
Strong acids completely dissociate (split) in solution, while weak acids partially dissociate.
How do chemical buffer systems maintain pH balance?
They neutralize excess acids or bases to keep pH levels relatively constant.
What are the consequences of increased body fluid pH?
can lead to alkalosis, which can cause muscle twitching and spasms.
What are the consequences of decreased body fluid pH?
can lead to acidosis, which can cause confusion and lethargy.
What triggers the thirst center in the hypothalamus?
Osmoreceptors detect changes in osmotic pressure due to water loss.
What role do stretch receptors in the stomach play in water intake regulation?
They send signals to inhibit the thirst center when the stomach is distended after drinking.
What is the effect of dehydration on extracellular fluid concentration?
increases the osmotic pressure of ECF, making it more concentrated.
What is the role of the kidneys in electrolyte regulation?
maintain electrolyte concentrations by adjusting reabsorption and excretion.
What are the vital functions of electrolytes?
Nerve impulse transmission, muscle fiber contraction, and maintenance of Resting Membrane Potential (RMP).
What hormone increases sodium reabsorption in kidney tubules?
Aldosterone.
What does aldosterone do to potassium levels?
It causes secretion (excretion) of excess potassium (K+) into the urine.
What hormone decreases calcium levels in the body?
Calcitonin.
How does calcitonin decrease calcium levels?
By targeting distal kidney tubules to secrete excess calcium (Ca++) into urine and inhibiting osteoclast activity.
What is the role of parathyroid hormone (PTH)?
It increases calcium levels by promoting reabsorption in kidney tubules, activating osteoclasts, and increasing dietary absorption.
What are the sources of hydrogen ions (H+) in the body?
Aerobic respiration of glucose, anaerobic respiration (lactic acid), fatty acid oxidation, sulfur-containing amino acids, and hydrolysis of phosphoproteins and nucleic acids.
What is the difference between strong and weak acids?
Strong acids completely dissociate (split) in water, while weak acids do not.
What is the bicarbonate buffer system?
A chemical buffer system that helps regulate pH by converting carbonic acid (H2CO3) to bicarbonate (HCO3-) and hydrogen ions (H+).
What is the phosphate buffer system?
A buffer system that involves the conversion of dihydrogen phosphate (H2PO4-) to hydrogen ions (H+) and hydrogen phosphate (HPO4^2-) to regulate pH.
What role do protein buffer systems play?
They involve plasma proteins and certain cellular proteins that help maintain pH by binding or releasing hydrogen ions.
How does the respiratory system regulate hydrogen ion concentration?
By excreting CO2, which affects carbonic acid levels and thus influences H+ concentration.
What happens during hyperventilation?
It decreases CO2 levels, which can lead to dangerous conditions if associated with breath-holding.
What is the chloride shift?
The exchange of chloride ions moving into red blood cells as bicarbonate ions leave, maintaining ionic balance.
What is the renal excretion of hydrogen ions?
The process by which kidney tubules secrete excess hydrogen ions into urine to help regulate pH.
What distinguishes chemical buffers from physiological buffers?
Chemical buffers act quickly, while physiological buffers take minutes to days to respond to pH changes.
What is acidosis?
A condition where pH drops below 7.4.
What is alkalosis?
A condition where pH rises above 7.4.
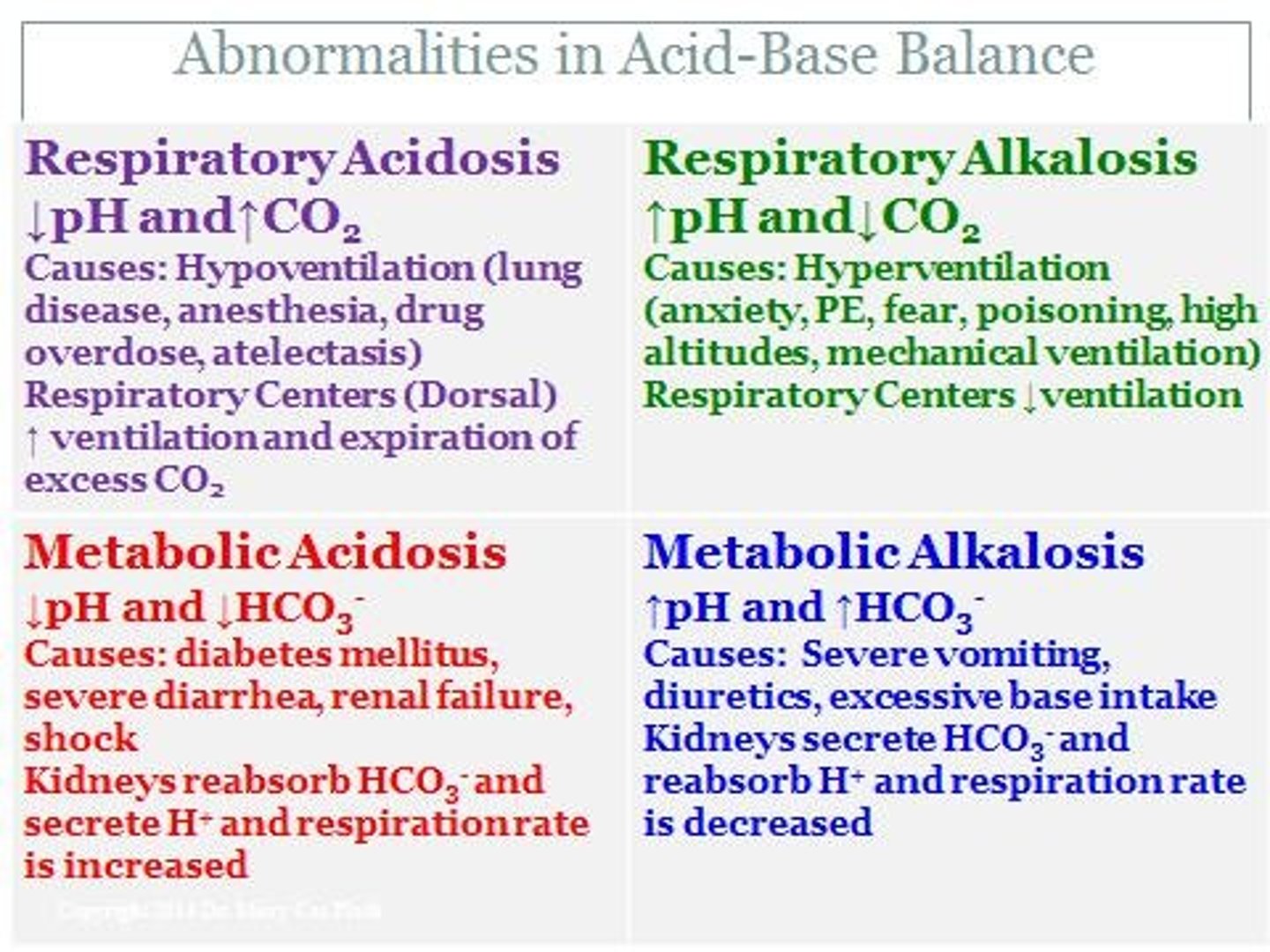
What causes respiratory acidosis?
Increased levels of carbon dioxide and carbonic acid.
What causes metabolic acidosis?
Accumulation of other acids or loss of bases.
What causes respiratory alkalosis?
Loss of carbon dioxide and carbonic acid.
What causes metabolic alkalosis?
Loss of other acids or gain of bases.
What is compensation in acid-base balance?
The process by which chemical buffering, respiratory mechanisms, and renal mechanisms return pH to normal.
What happens if one compensatory mechanism fails?
Another mechanism takes over to maintain pH balance.