Covalent and Non-covalent Bonding
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
Do atoms want a complete outer valence shell?
Yes
Why do bonds form?
Because bonded atoms are at lower energy than isolated atoms (more stable)
What is ionisation?
Loss or gain of electrons to form ions.
What is ionic bonding?
Electrostatic attraction between oppositely charged ions
Example of Ionic Bonding
NaCl
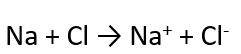
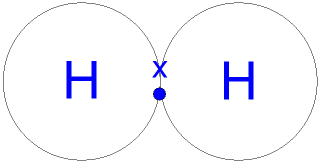
What is a covalent bond?
A bond formed by sharing a pair of electrons between two atoms.
What happens when covalent bonds form?
Atomic orbitals overlap to form a molecular orbital of lower energy.
Example of Covalent Bonding
H2
The 1s orbitals of both atoms merge into a single bond orbital that contains both electrons
What is bond dissociation energy?
The energy required to break a bond.
The greater the orbital overlap, the stronger the bond
Name 2 types of chemical covalent bonds:
Sigma (σ) bonds
Pi (π) bonds
What is a sigma (σ) bond?
A strong covalent bond formed by head-on overlap of orbitals.
Single bonds are sigma (σ) bonds.
What is a pi (π) bond?
A covalent bond formed by side-on (lateral) overlap of p orbitals.
π bonds occur in addition to an existing σ bond (forming double and triple bonds).
Why are π bonds weaker than σ bonds?
Less effective orbital overlap.
Why can’t double bonds freely rotate?
Rotation would break the π bond.
What is the consequence of restricted rotation?
Geometric (cis–trans / E–Z) isomerism.
What is a lone pair?
A pair of electrons not involved in bonding.
Why does NH3 have bond angles of 107° instead of 109.5° (tetrahedral)
The lone pair exerts a slightly greater repulsion than the sigma bonds, compressing the bond angle from 109.5° to 107°
Why is the bond angle for H2O 104.5°?
There are 2 lone pairs on the O
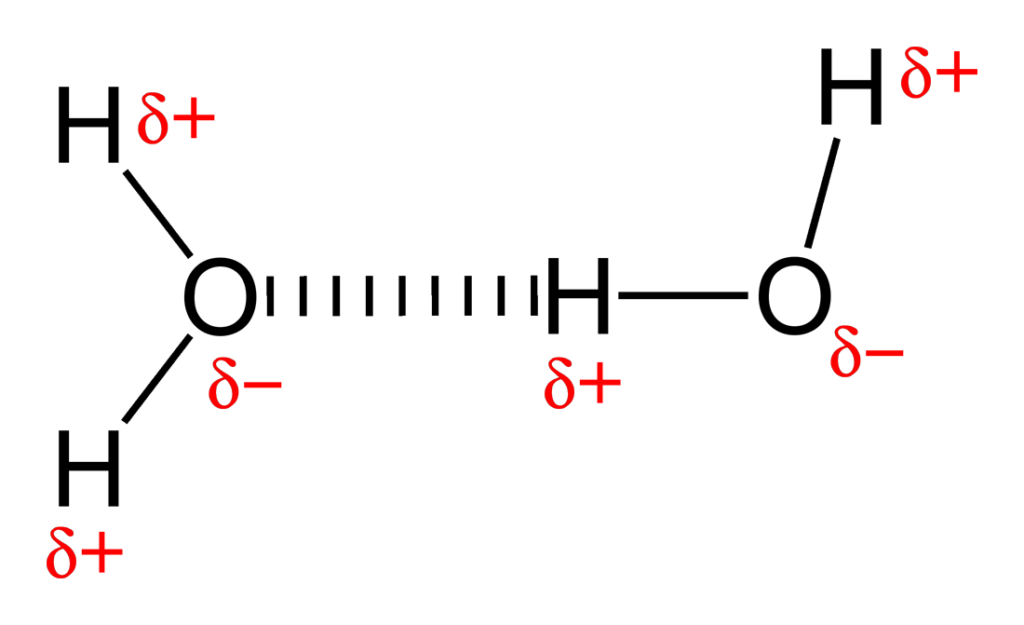
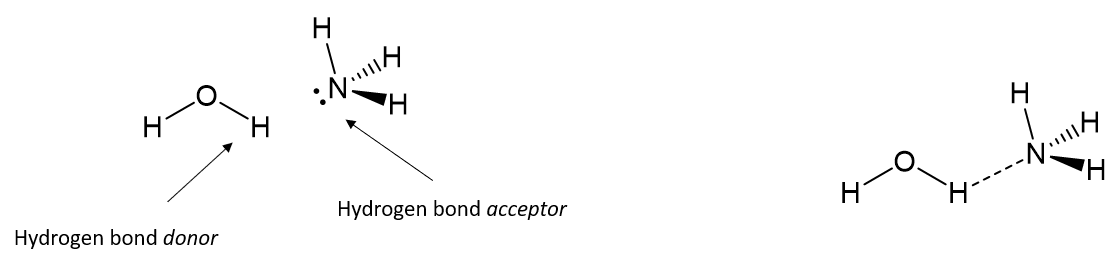
What is a hydrogen bond?
A partially electrostatic attraction between a H that is bonded to N/O/F and a lone pair on another atom.
Most electronegative atoms
N, O, F
What is a hydrogen bond donor?
The group providing the H (e.g. O–H, N–H).
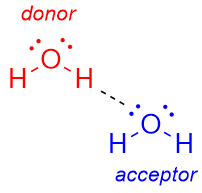
What is a hydrogen bond acceptor?
The atom with the lone pair (e.g. O, N).
Example of Hydrogen Bonding
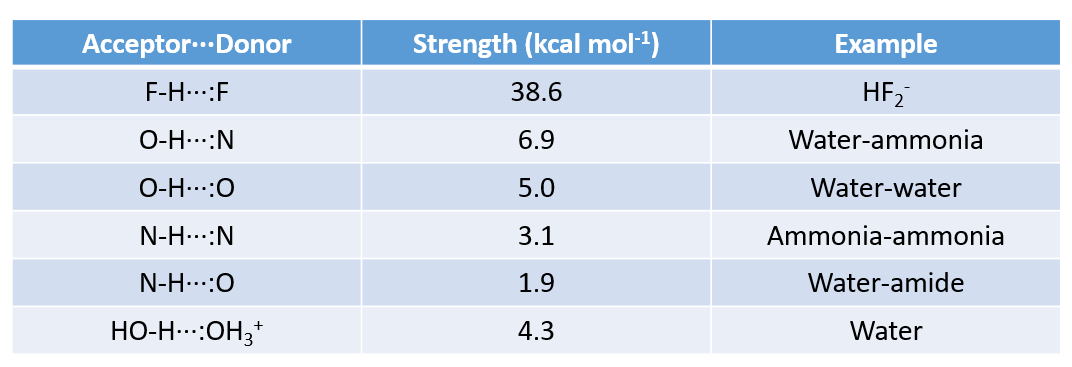
Strength of Hydrogen Bonds
What does hydrogen bonding do in small molecules?
Increases the melting point
Increases the boiling point
Increases the solubility
Increases the viscosity
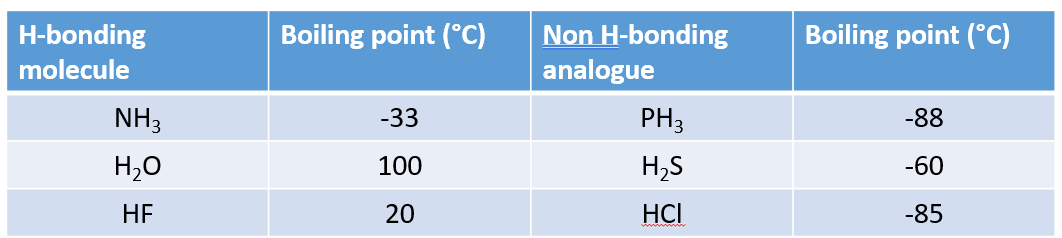
Why does ice float on water?
H-bonded lattice in ice is less dense than liquid water.
What are Van der Waal forces?
Weak intermolecular attractions that act between all atoms and molecules.
Weakest type of intermolecular force
Present in all molecules
Strength increases with:
More electrons / larger atoms
Greater surface area
Name the three types of Van der Waals forces:
Dipole-dipole
Dipole-induced dipole
London forces
1) Van der Waals forces (dipole-dipole)
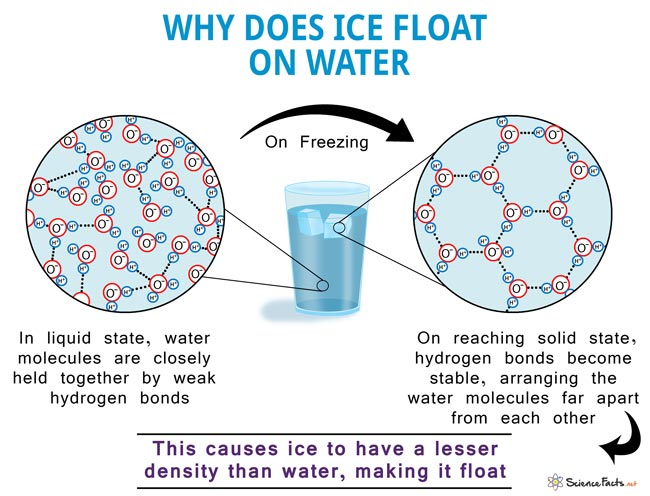
Some electrically neutral molecules may exhibit permanent electric dipoles.
For instance consider water – a bent molecule with a bond angle of around 105°.
The oxygen atom is always somewhat negatively charged and the hydrogen atoms (side) somewhat positive
These dipoles have a tendency to align, and this results in a net attractive force.
Note – this is a lot smaller than any H-bonding interaction present (e.g. in water).
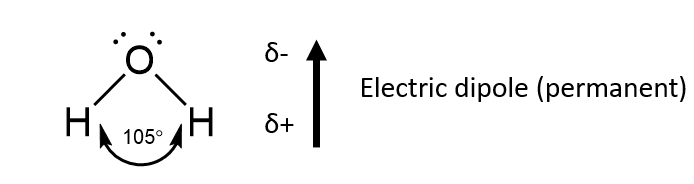
2) Van der Waals forces (dipole-induced dipole)
Molecules with a permanent dipole may temporarily distort the electric charge in a nearby molecule (polar or non-polar).
The extra attraction is between the permanent dipole and the ‘induced’ dipole on the nearby molecule.
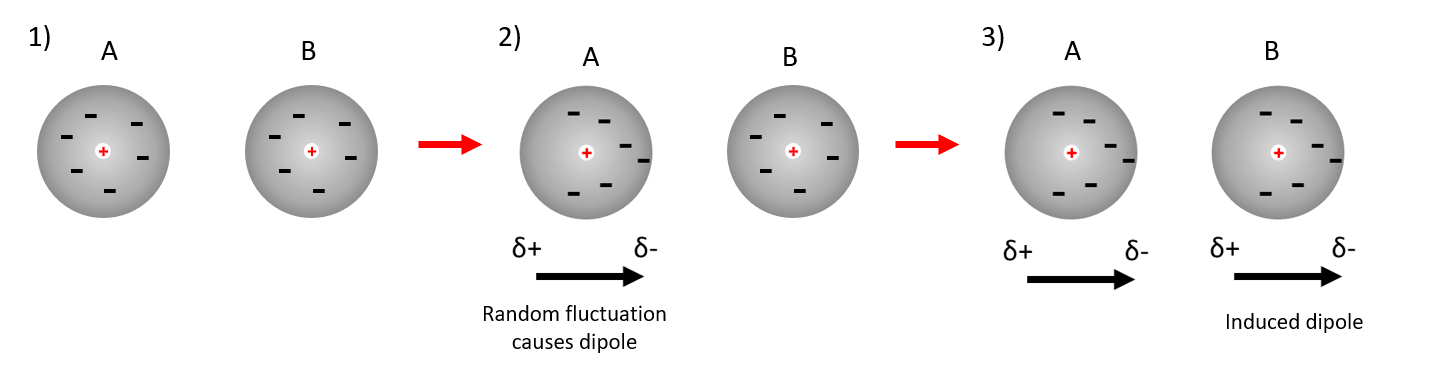
3) Van der Waals forces (London forces)
Thirdly in molecules with no permanent dipole, temporarily, dipoles result from the random electron motion within the atoms.
At any given time the centre of positive charge arising from the nucleus and the centre of negative charge arising from the electrons are unlikely to coincide.
This leads to instantaneous, but short lived dipoles, even though over time the average polarisation is zero.
The resulting instantaneous dipoles are too short lived to align with other molecules to give an attractive force, however they can induce polarization in adjacent molecules.
These specific interactions (forces) are known as London forces, or dispersion forces.
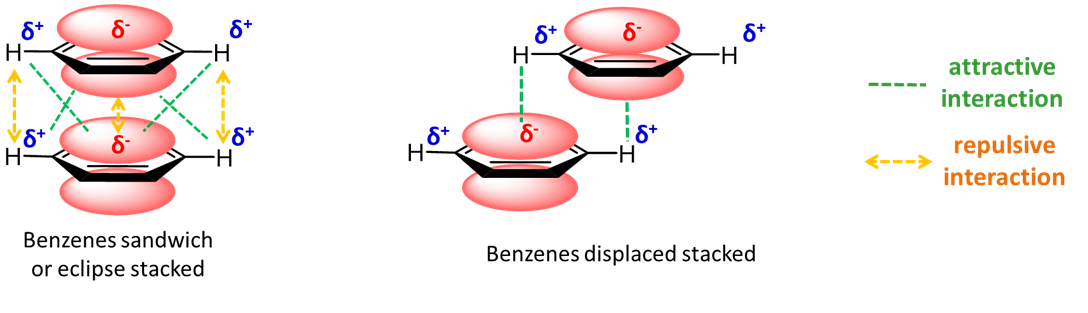
What is π-stacking?
Non-covalent attraction between aromatic rings.
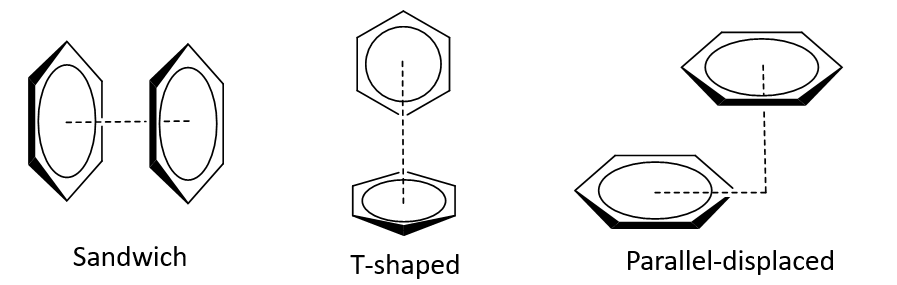
What are the 3 recognised arrangements of pi stacking (π- π interactions)?
Sandwich
T-shape
Parallel-displaced
Why is π-stacking biologically important?
Stabilises protein structures (aromatic amino acids).
What drives hydrophobic interactions?
Minimising disruption of water’s hydrogen-bond network.
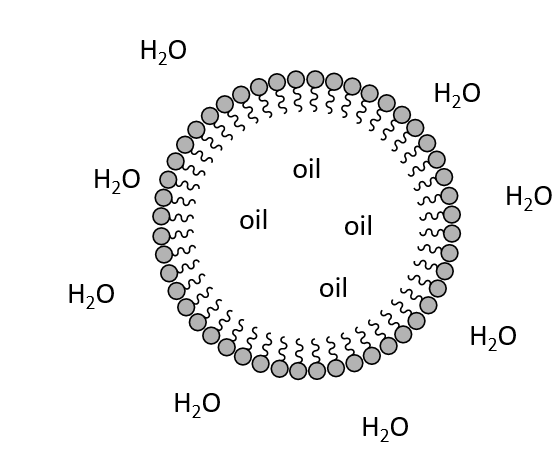
What is an amphiphile?
Molecules with both hydrophobic and hydrophilic regions
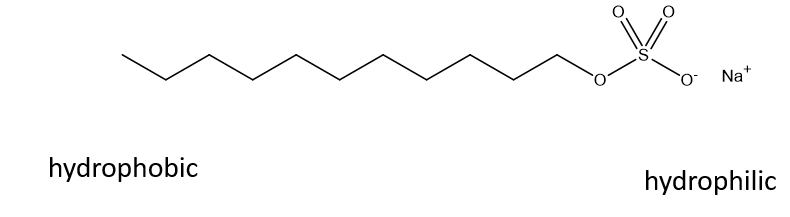
What is a micelle?
An aggregate with hydrophobic core and hydrophilic surface.
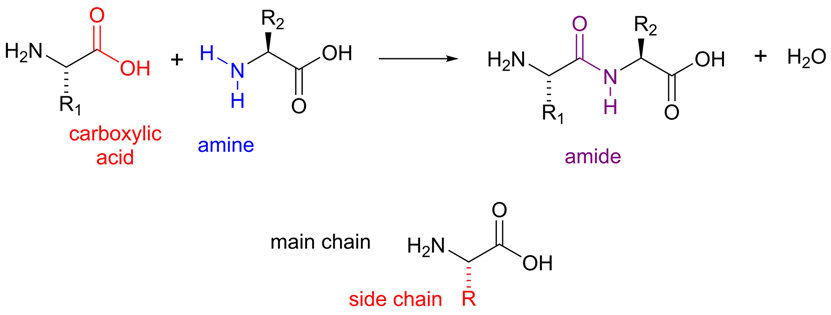
Primary structure of Protein
Amino acid sequence.
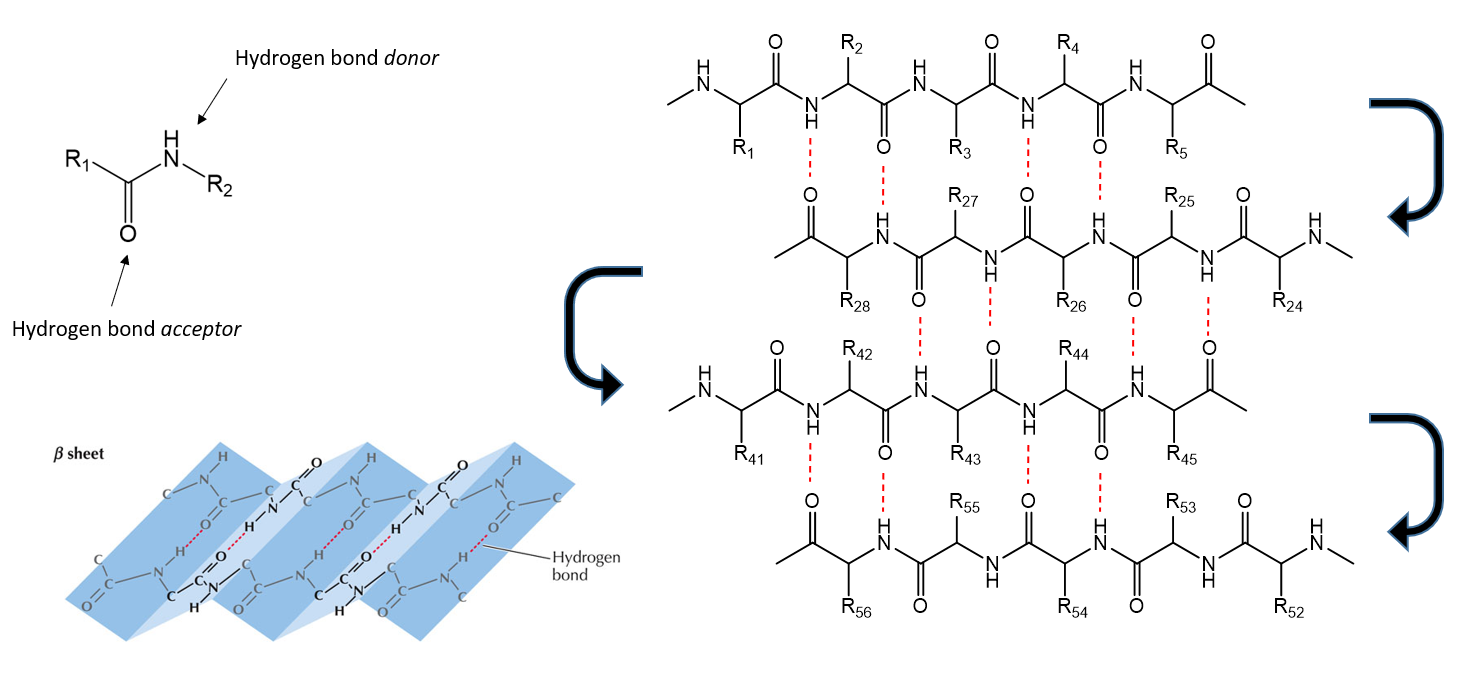
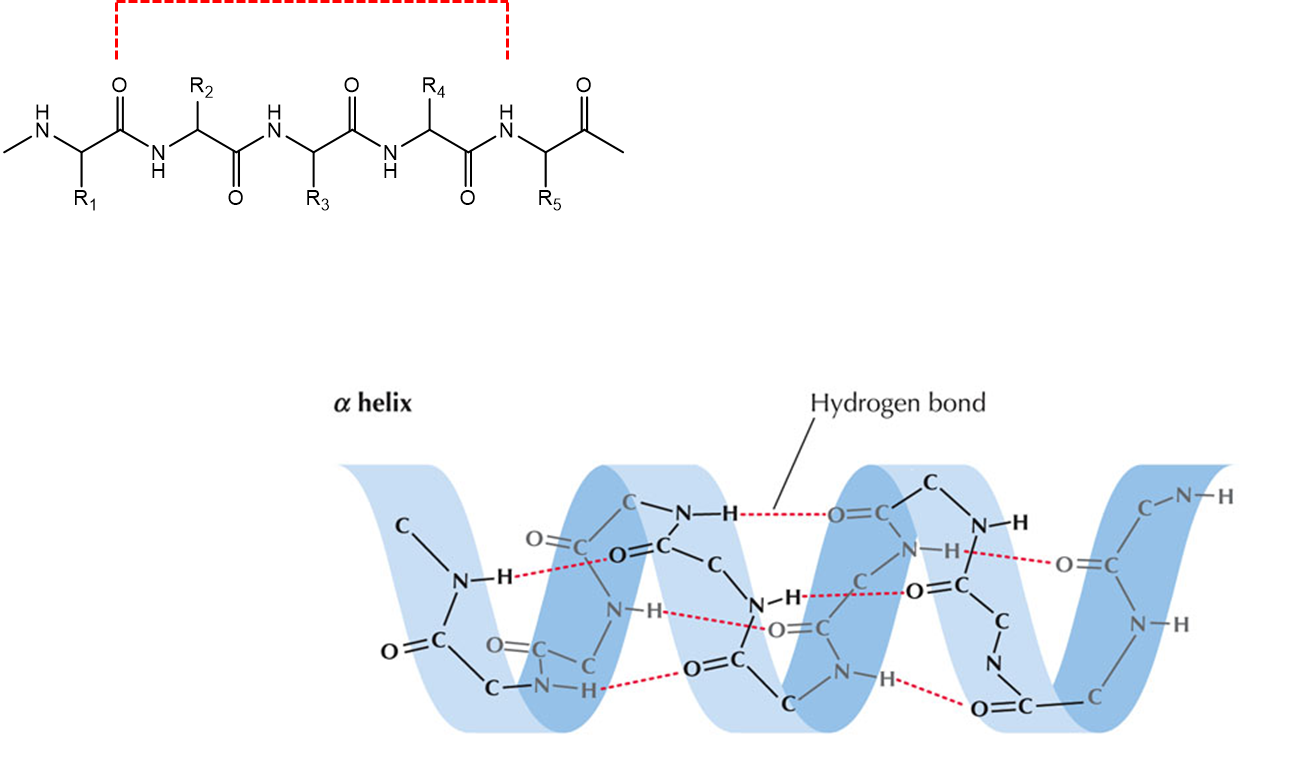
Secondary structure of Protein
α-helix and the β-sheet, stabilised of hydrogen bonds between the main-chain peptide groups.
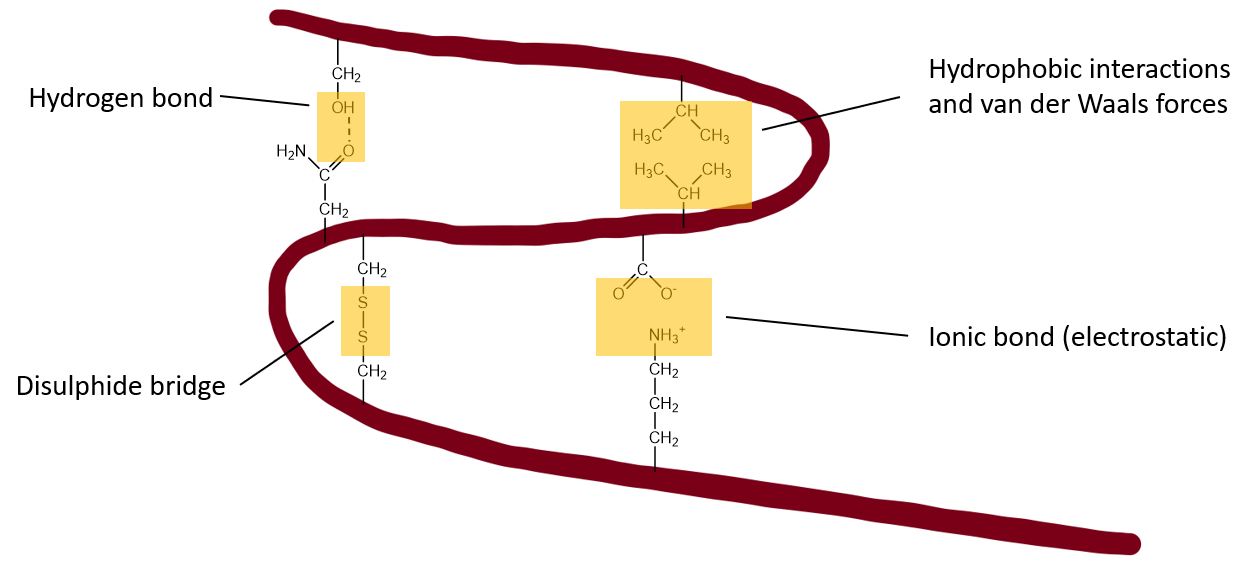
Tertiary structure of Protein
- Overall 3D fold of one polypeptide chain.
The α-helices and the β-sheets are folded into a compact globular structure, driven by the hydrophobic interactions
There is further stability by salt bridges, hydrogen bonds, the tight packing of side chains (van der Waals) and disulphide bonds.
What stabilises tertiary structure of a protein?
Hydrophobic interactions
H-bonds
Salt bridges
Van der Waals
Disulphide bonds
π-stacking
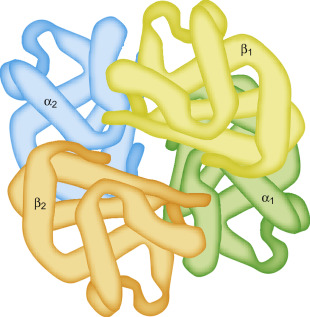
Quaternary structure of Protein
Refers to assemblies of two or more individual polypeptide chains into one single functional unit
Stabilized in a similar way to the tertiary structure
The primary structure of a protein is reported starting at which end?
Amino terminal (N) end
Protein secondary structure – α-helix
The carbonyl of residue n interacts with the amide proton of residue n+4.
Protein secondary structure - β-sheets