GV-5 Inducible Transcription Factors 1
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
83 Terms
Why do we need to tightly control gene transcription processes?
Bc too much cell death can mean neurodegeneration, or too much growth can mean cancer.
What are diseases/health conditions often caused by?
Aberrant protein function
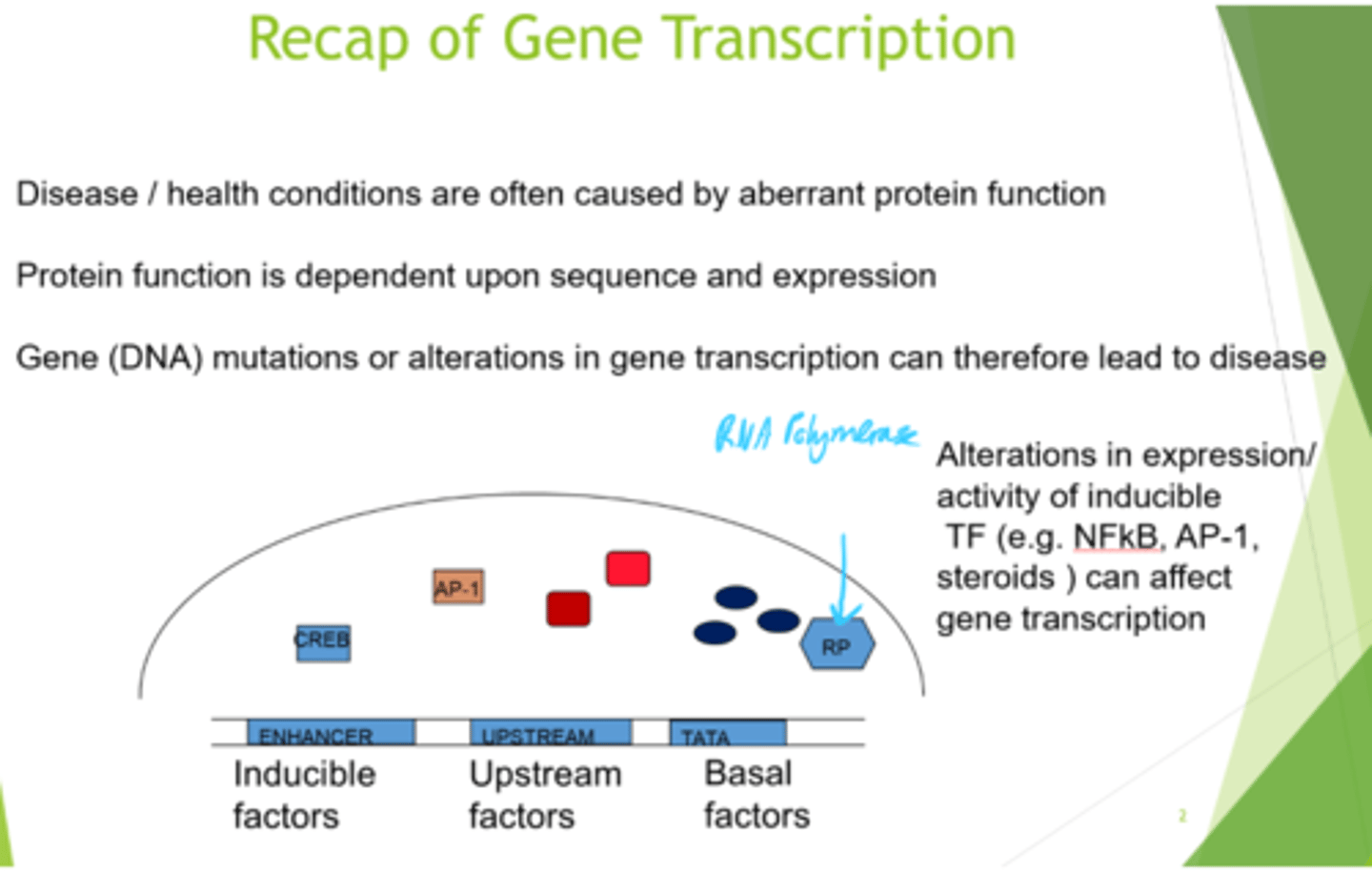
What is protein function dependent on?
Gene sequence and expression
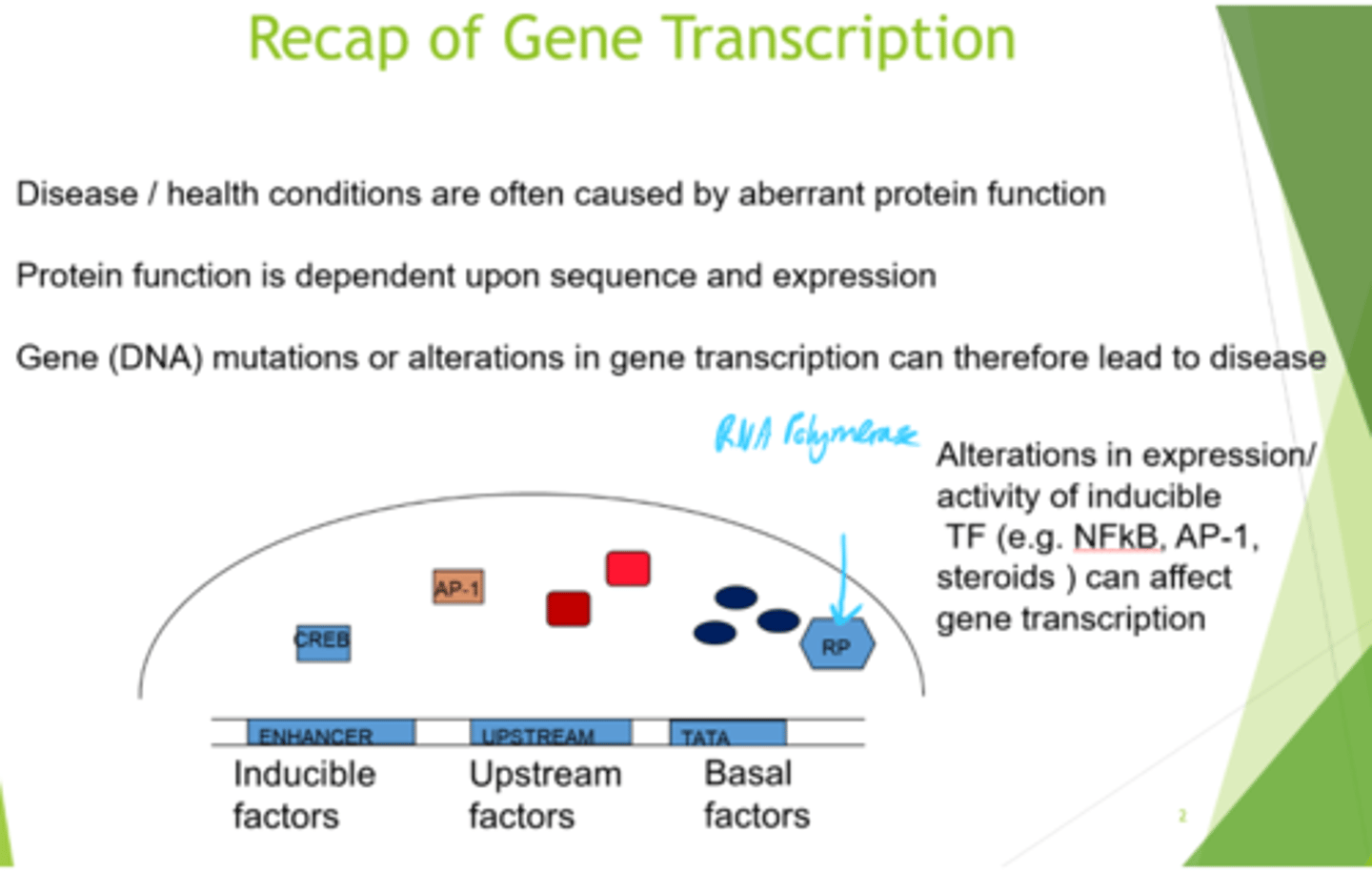
What can transcription factors be grouped into?
Inducible, upstream or basal factors.
How are inducible factors activated/induced?
By phosphorylation and dimerisation
How can we get specificity by utilising inducible factors?
By combination - can mix and match homo + heterodimers, each of which binds to dif sequences in the enhancer, and switches on dif gene transcription.
What do basal factors bind to?
(as does RNA polymerase).
TATA box
What is NFKB (NF kappa B)?
Nuclear Factor of Kappa (light chain in B cells.
The light chain of antibodies comes in 2x varieties. What are these called?
Kappa and LAMDA
What is NFKB generally associated with?
Activation of pro-inflammatory genes.
Lots of inflammatory disorders are triggered by activation of NFKB.
What does all NFKB contain?
A conserved sequence aka Rel domain.
What does the Rel domain sequence do in NFKB?
Regulates dimerisation and nuclear localisation.
Where is NFKB found in resting cells?
- In cytoplasm.
- Moves to nucleus and dimerises, binding to DNA sequence and drives transcription.
How is NFKB inhibited?
By IKB (Inhibitor of Kappa B) binding across Rel domain.
What is the transcription factor NFKB involved in?
Immune responses and inflammation.
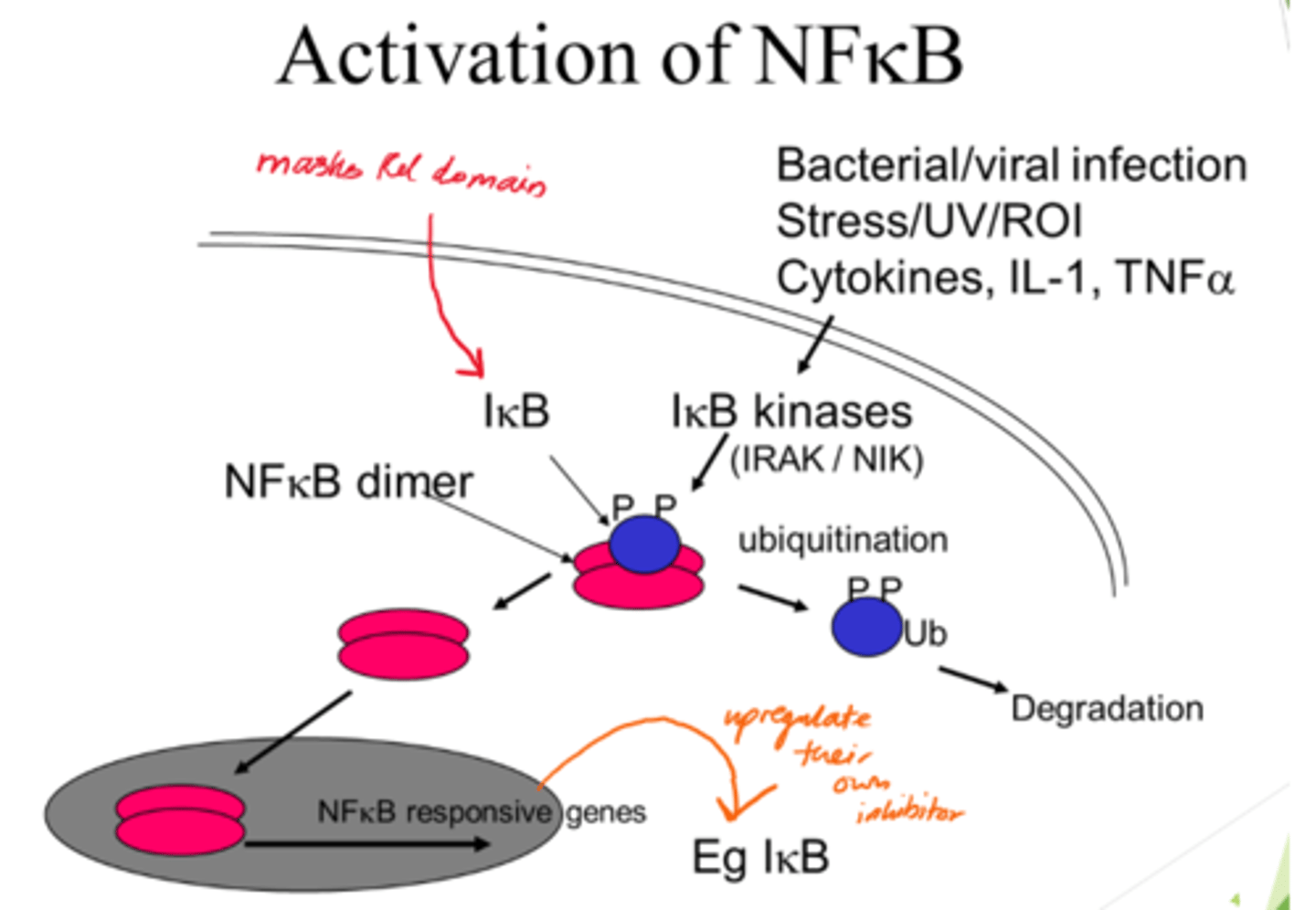
What triggers the pathway to activate NFKB?
Stresses to cells eg UV/ROS, infections, cytokines (esp. pro-inflammatory).
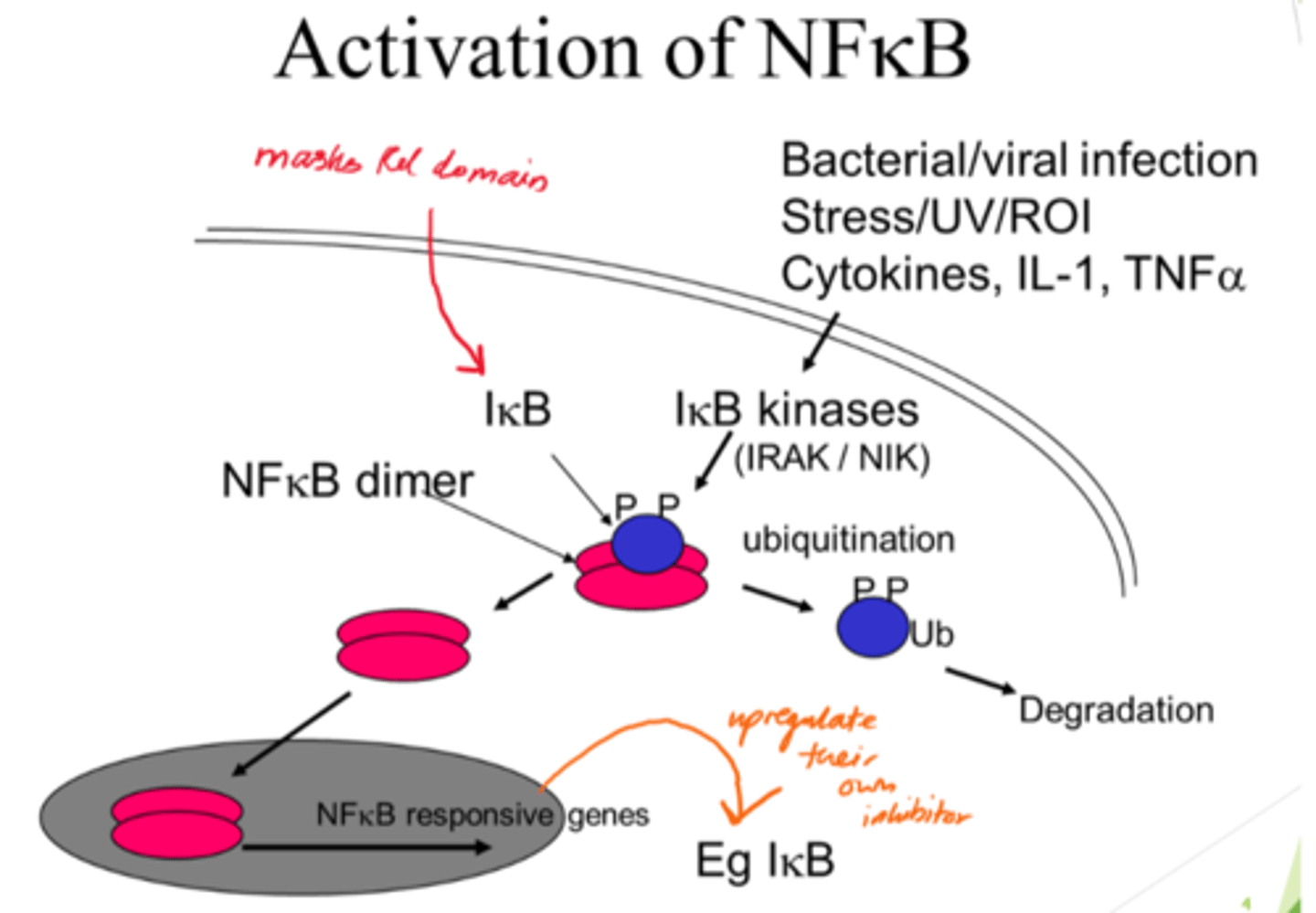
Describe NFKB activation
- NFKB dimer initially inactive when bound to IKB proteins.
- IKB kinases acivated and phosphorylate IBK.
- Ubiquitination: Phosphorylated IKB proteins are ubiquinated, marking them for degradation.
- IKB degradation frees NFKB dimers so they can enter nucleus.
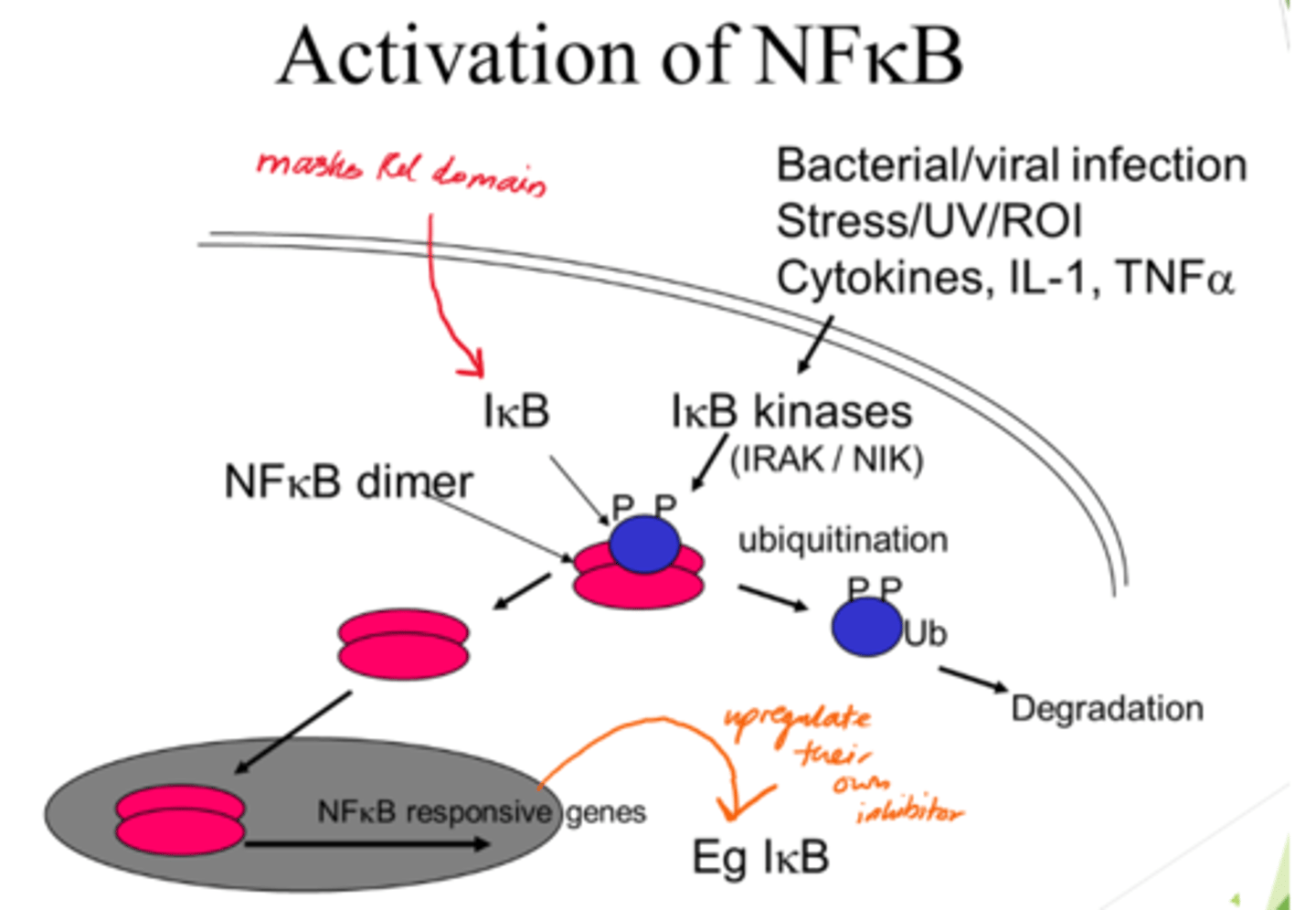
What is ubiquitination?
Marking a protein for degradation.
What is the outcome of NFKB activation?
- Once in nucelus, NFKB dimers activate gene transcription.
- IKB gene also activated for -ve feedback.

What does the masked Rel domain refer to?
The part of NFKB that's typically inactivated by IKB
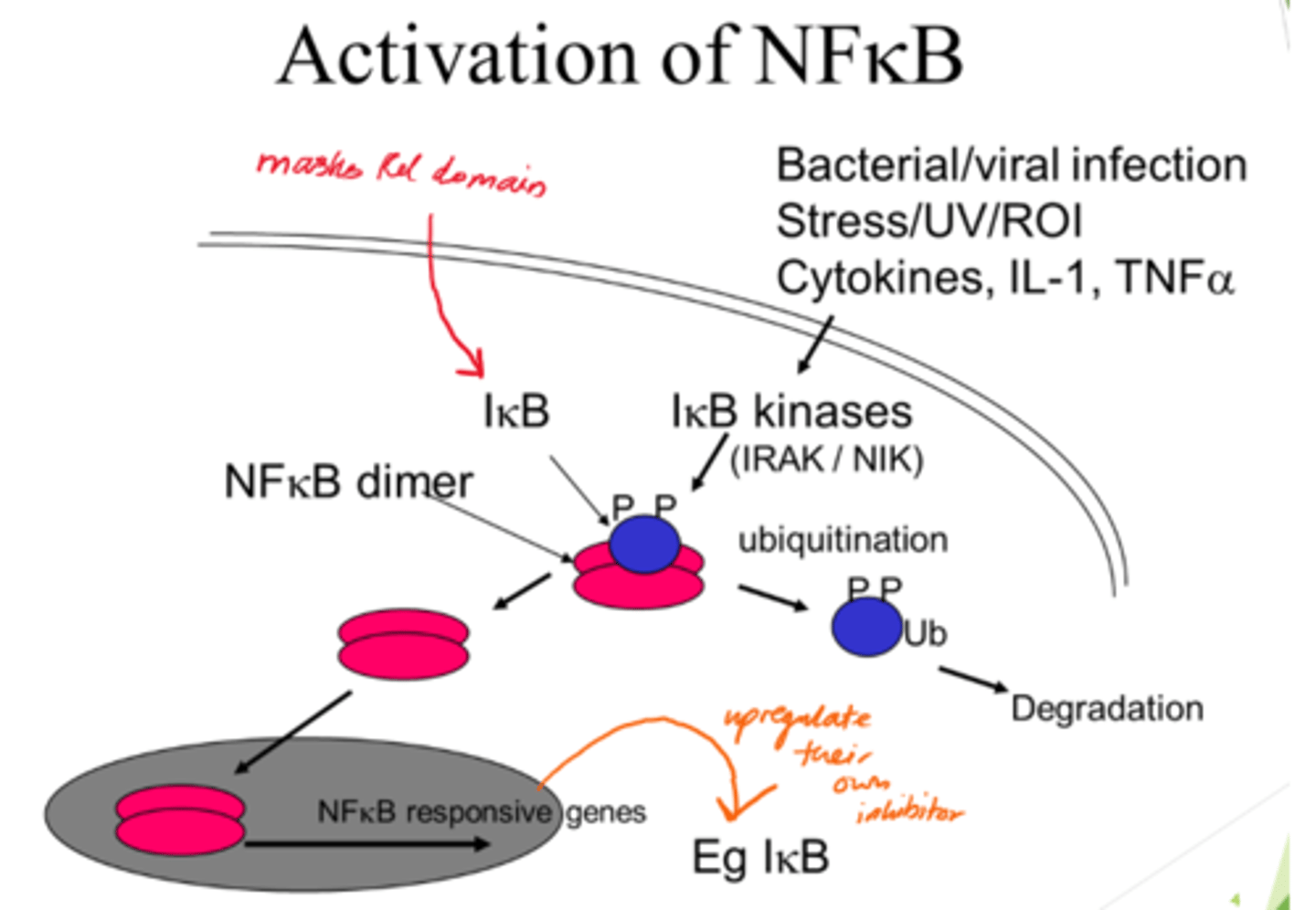
How can we inhibit the NFKB activation pathway?
- Inhibit kinases (so IKB stays bound to NFKB).
- Block nuclear translocation.
- Block DNA binding.
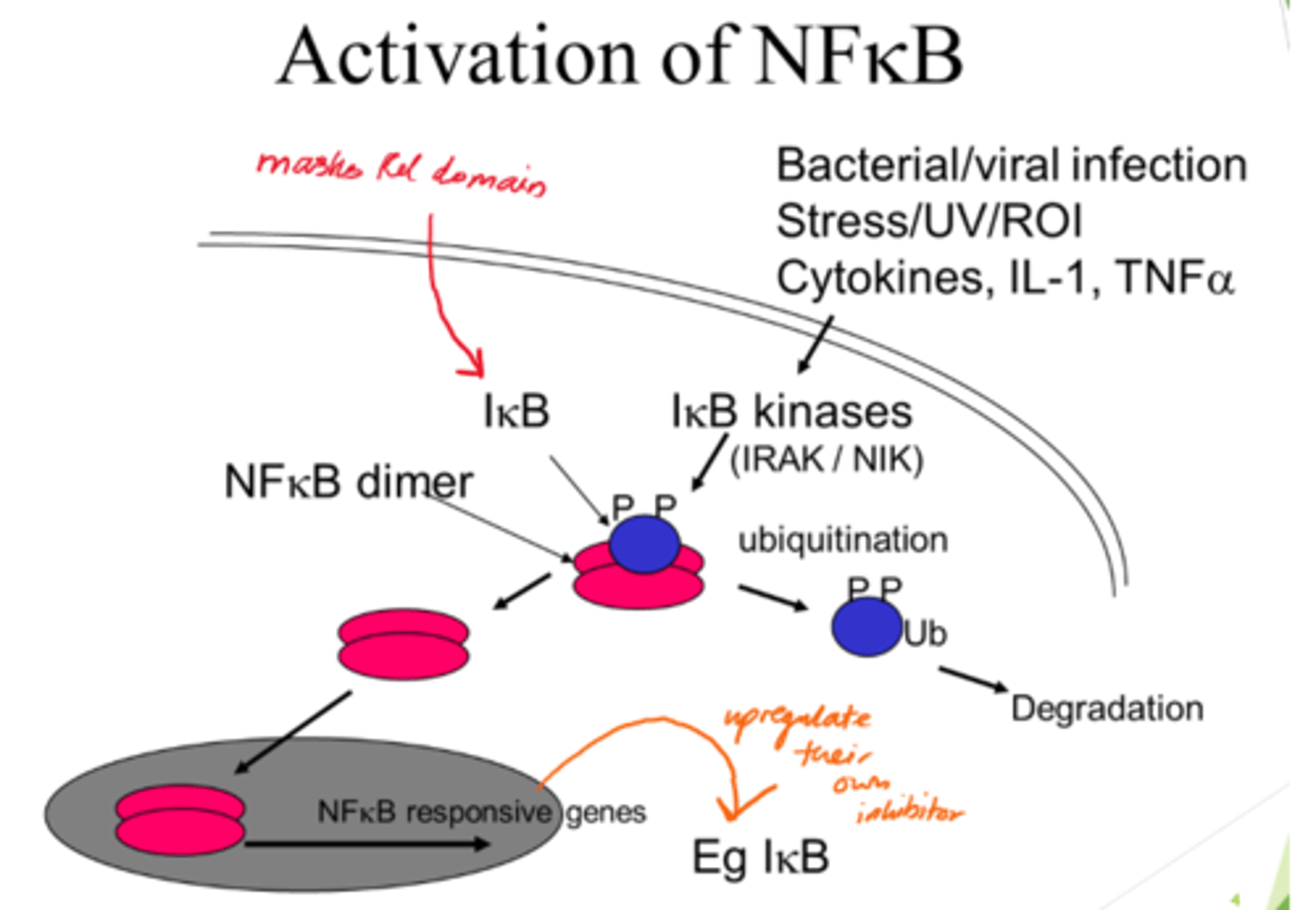
What happens when IKB is degraded?
NFKP is freed up to translocate into the nucleus.
Binds to promoter region of NFKB-responsive genes.
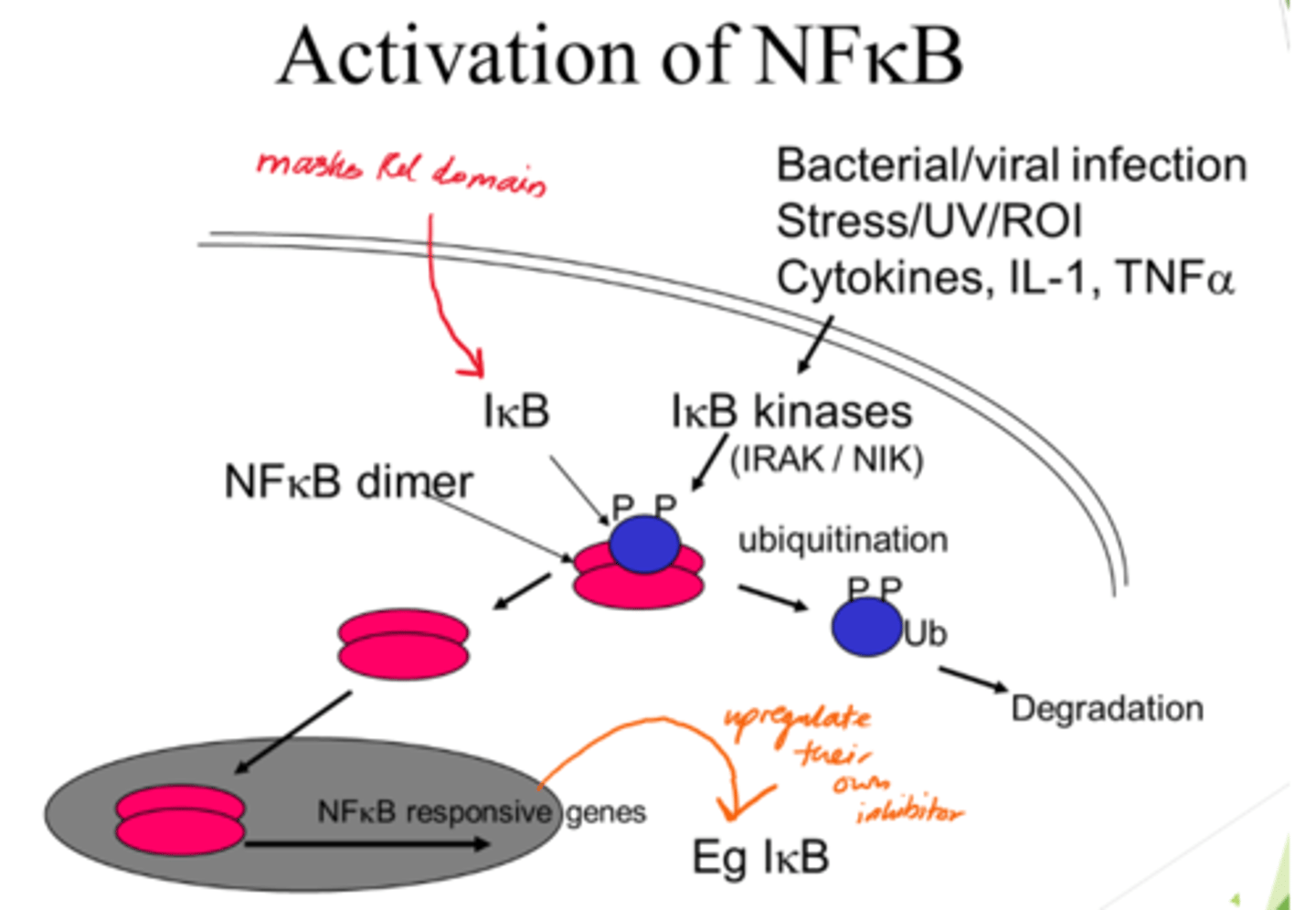
What is Respiratory Distress syndrome (from covid infection) caused by?
High levels of NFKB induced pro-inflammatory cytokines.
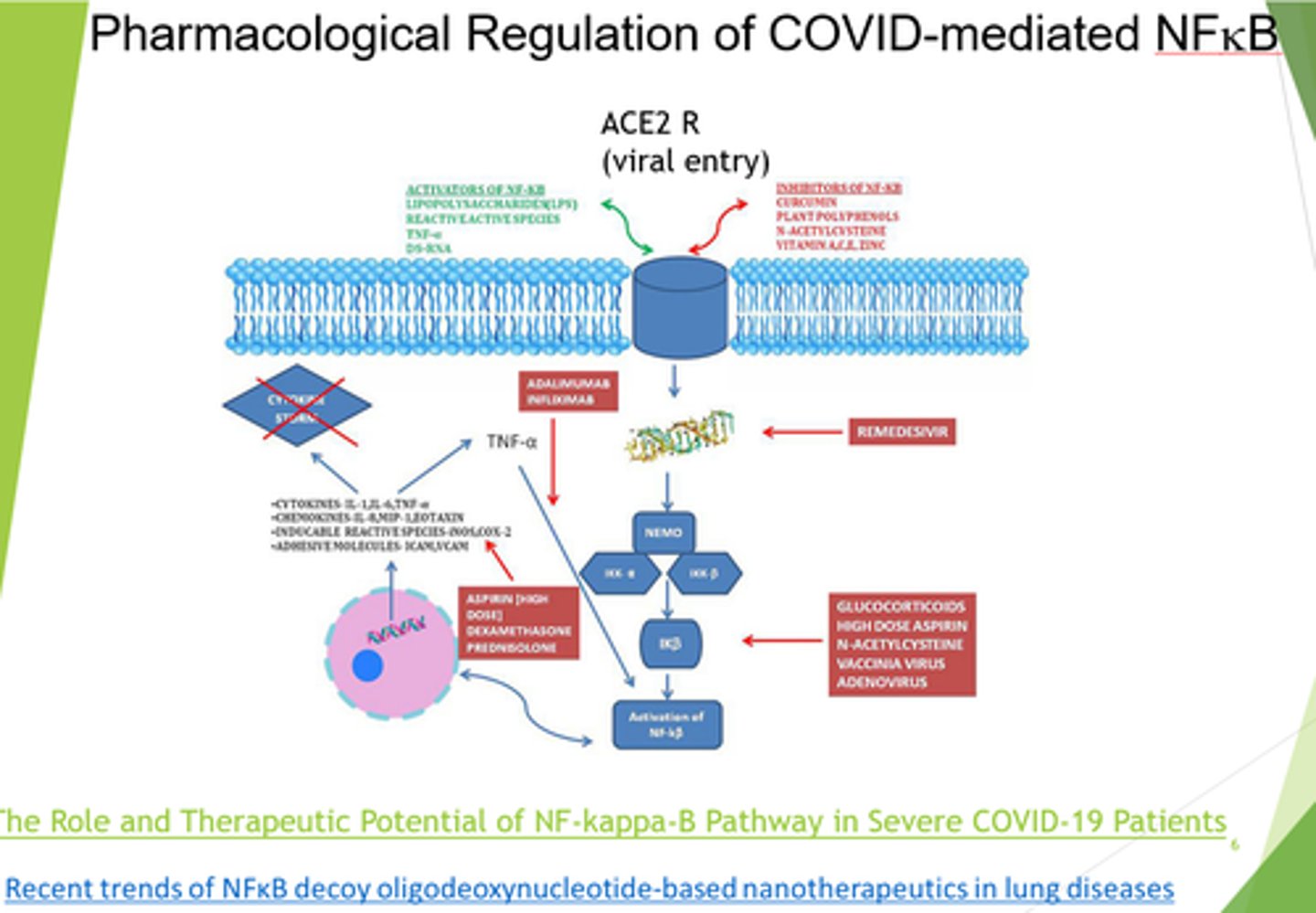
What diseases is NFKB a major inducer of?
Asthma and COPD-related inflammation.
Through which receptor is covid entry into cells mediated by?
ACE2
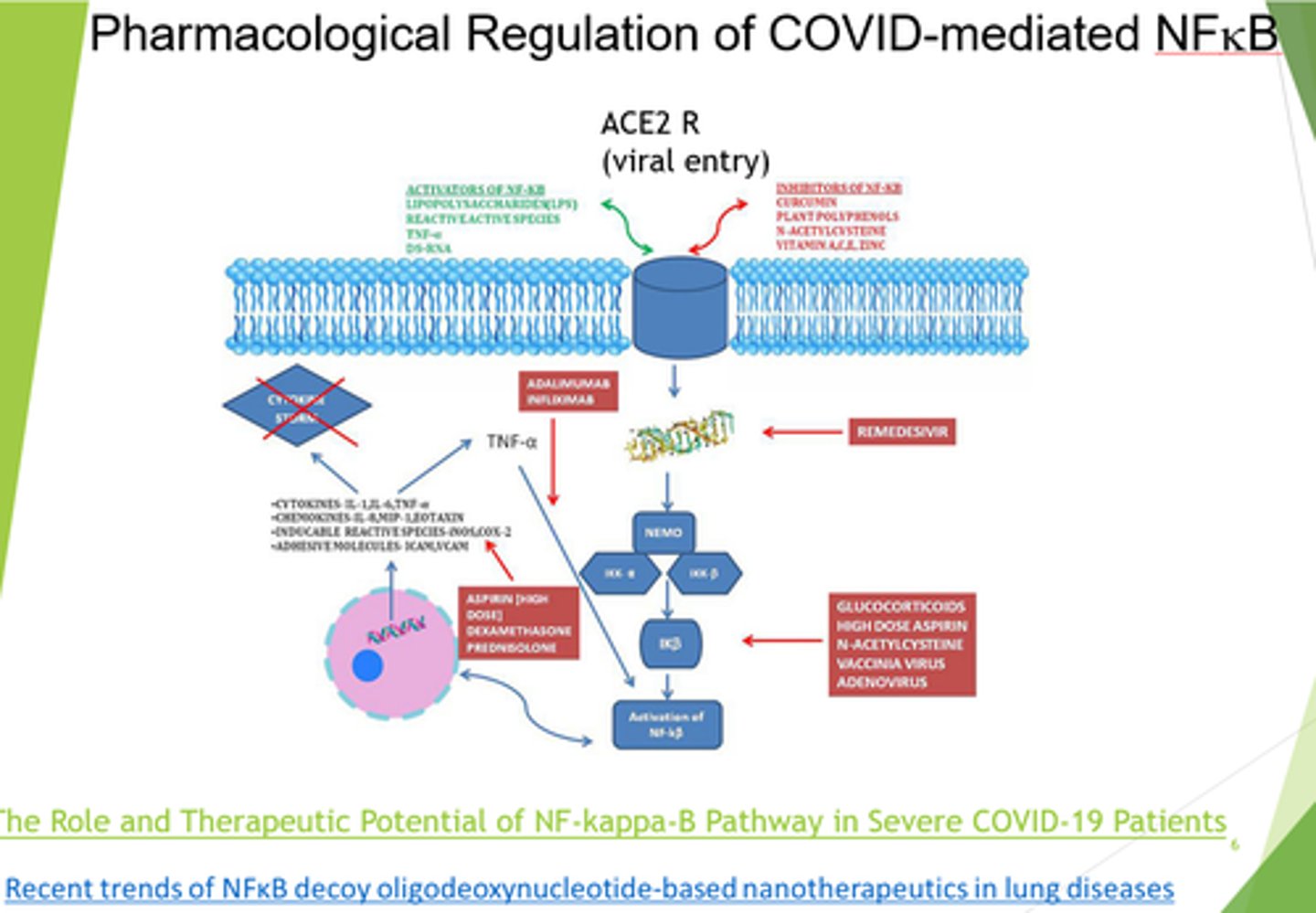
What can we target in severe covid/respiratory distress syndrome?
- Block ACE2 receptors.
- Inhibit NFKB.
- Inhibit viral replication.
- Anti-TNF therapies.
What does AP-1 (Activator Protein 1) do?
- Regulates immediate early response genes.
- Regulates cellular function eg cell growth.

What are the families called that make up the AP-1 dimer?
Fos and Jun families make up AP-1 dimer.
What are the levels of Fos in resting cells like?
Very low/non-existent.
When are Fos/Jun expressed?
- Fos is only transcribed when needed to make AP-1 to drive growth.
- Jun is constit expressed.
What does Jun require to make it active so it can dimerise?
Phosphorylation
What does the formation of AP-1 protein require?
- Transcription of Fos family member.
- Phosphorylation of Jun family member.
What is regulation of the C-Fos promoter important for?
Gene expression in response to growth signals
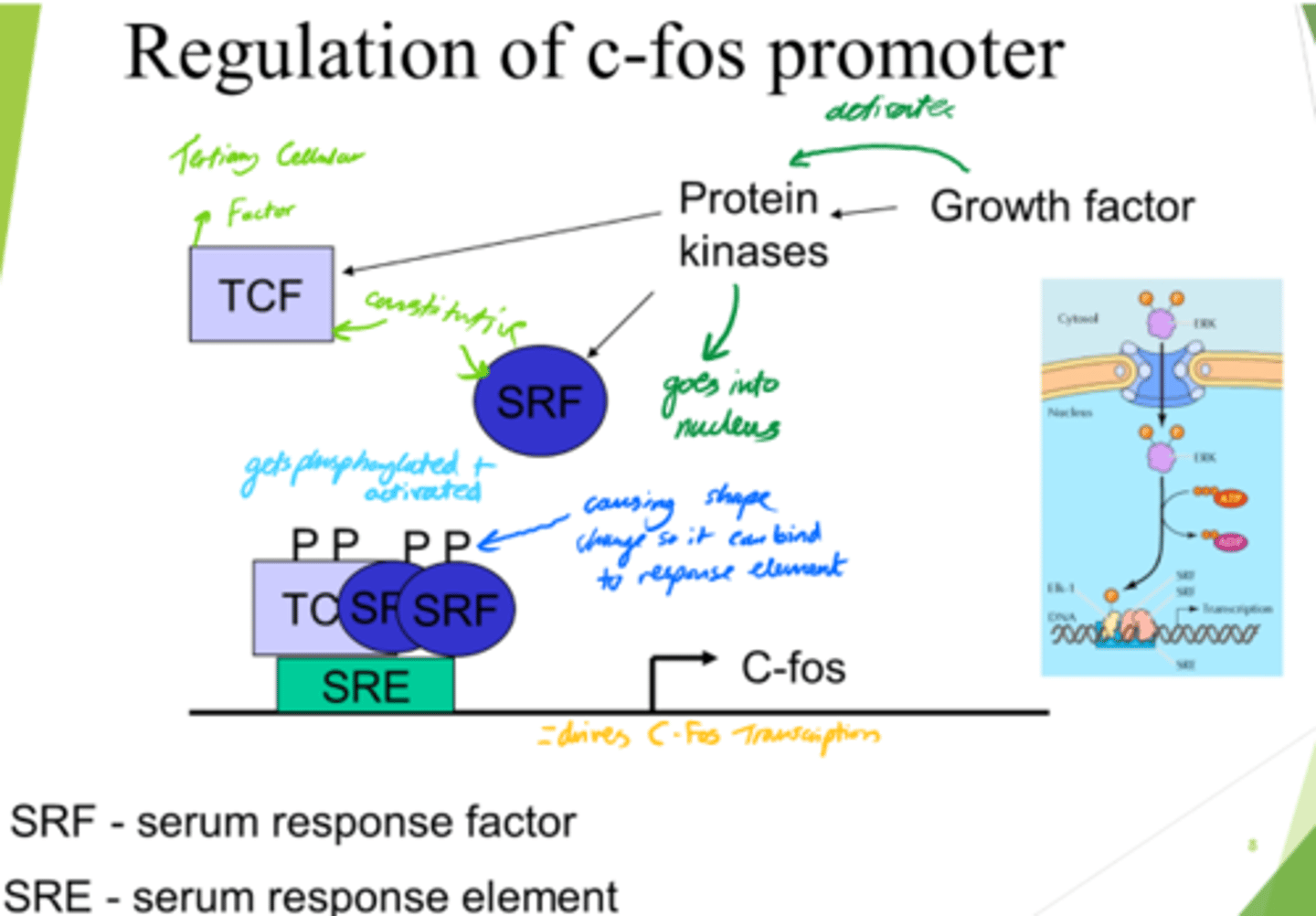
What do growth factors do?
Activate protein kinases, leading to downstream signalling.
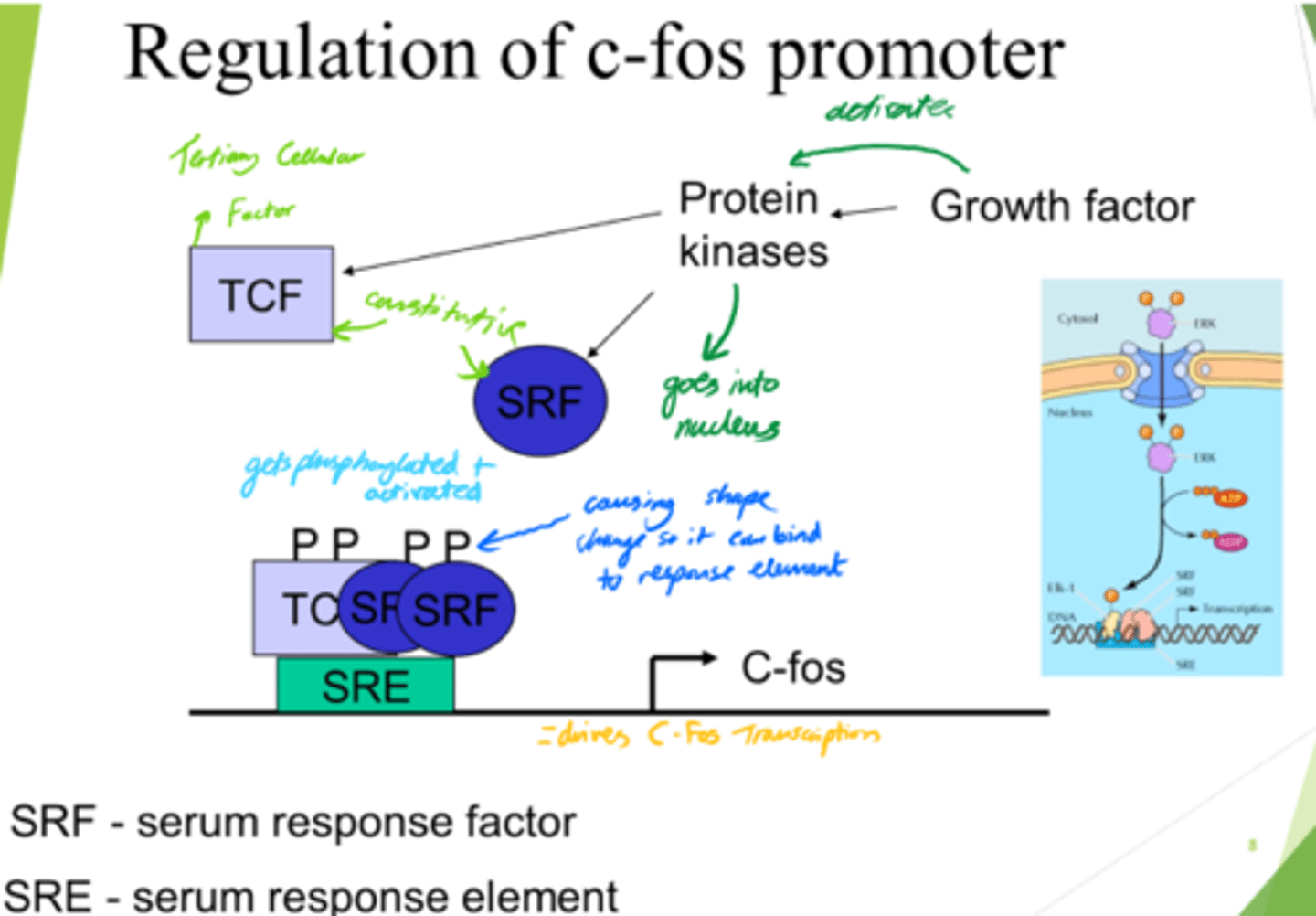
What is SRF?
Serum Response Factor
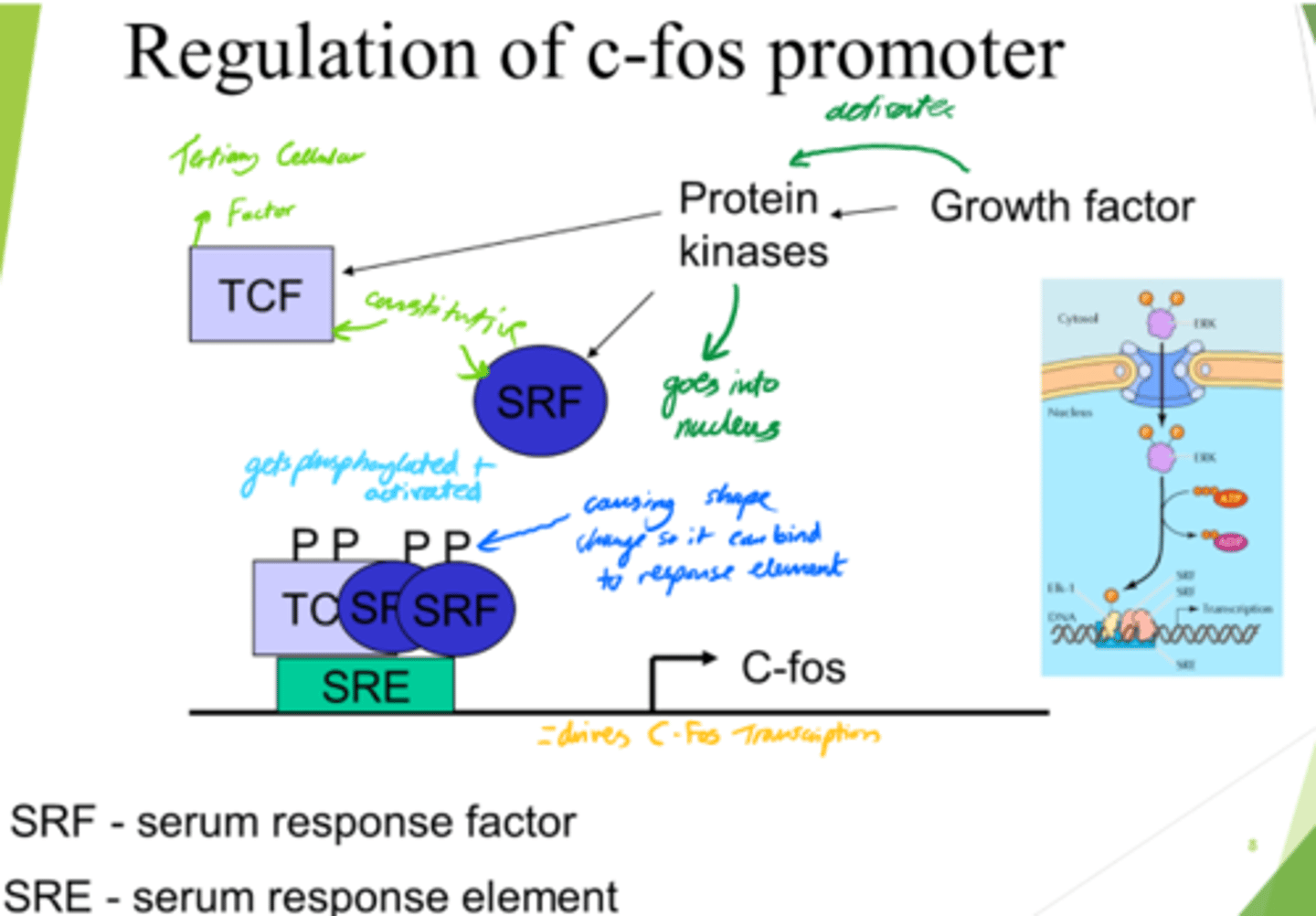
How is SRF activated?
(Serum Response Factor)
via Phosphorylation
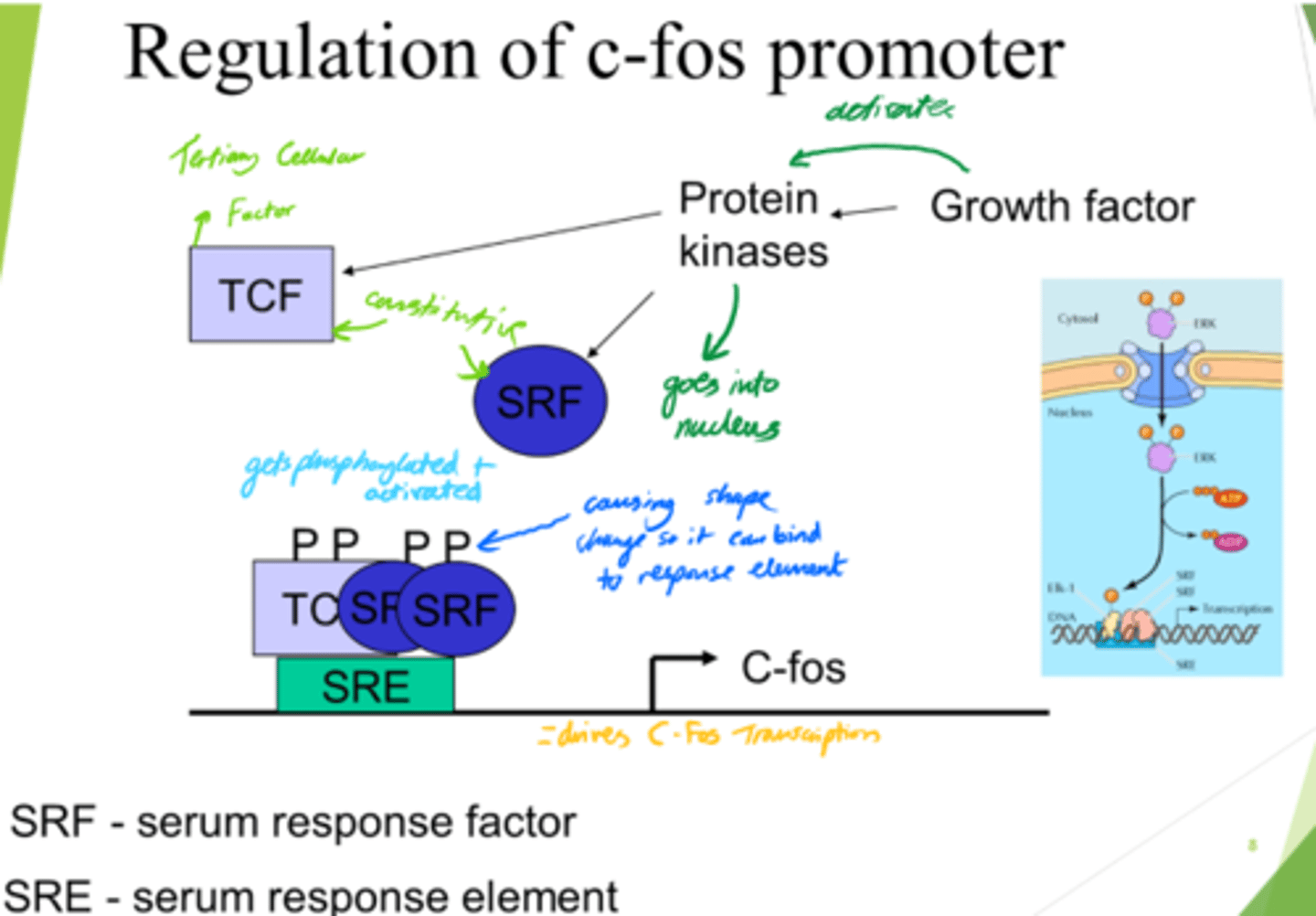
What does SRF do?
- Enters nucleus.
- Conformation change.
- Binds to SRE (Serum Response Element).
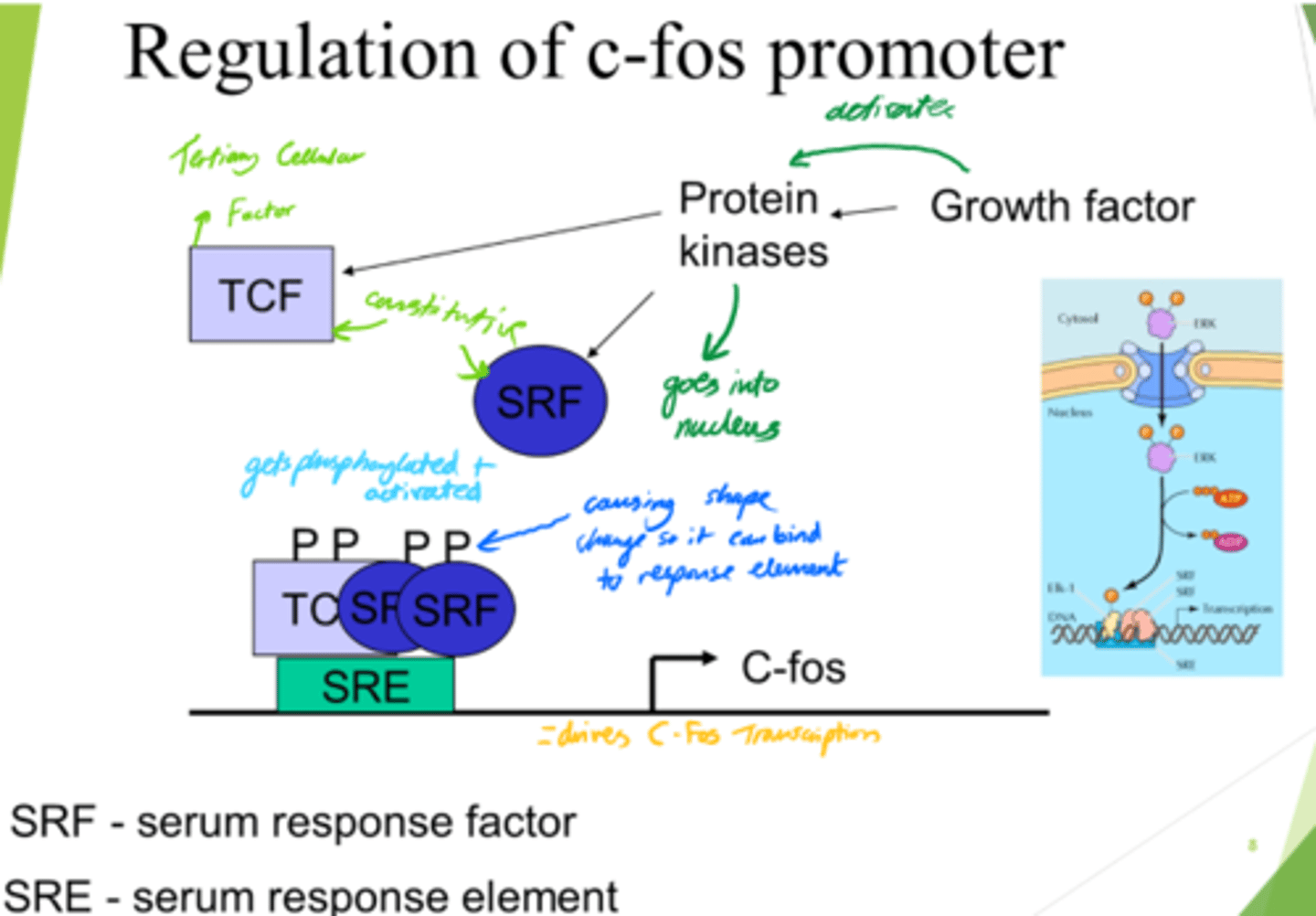
What is SRE?
Serum Response Element
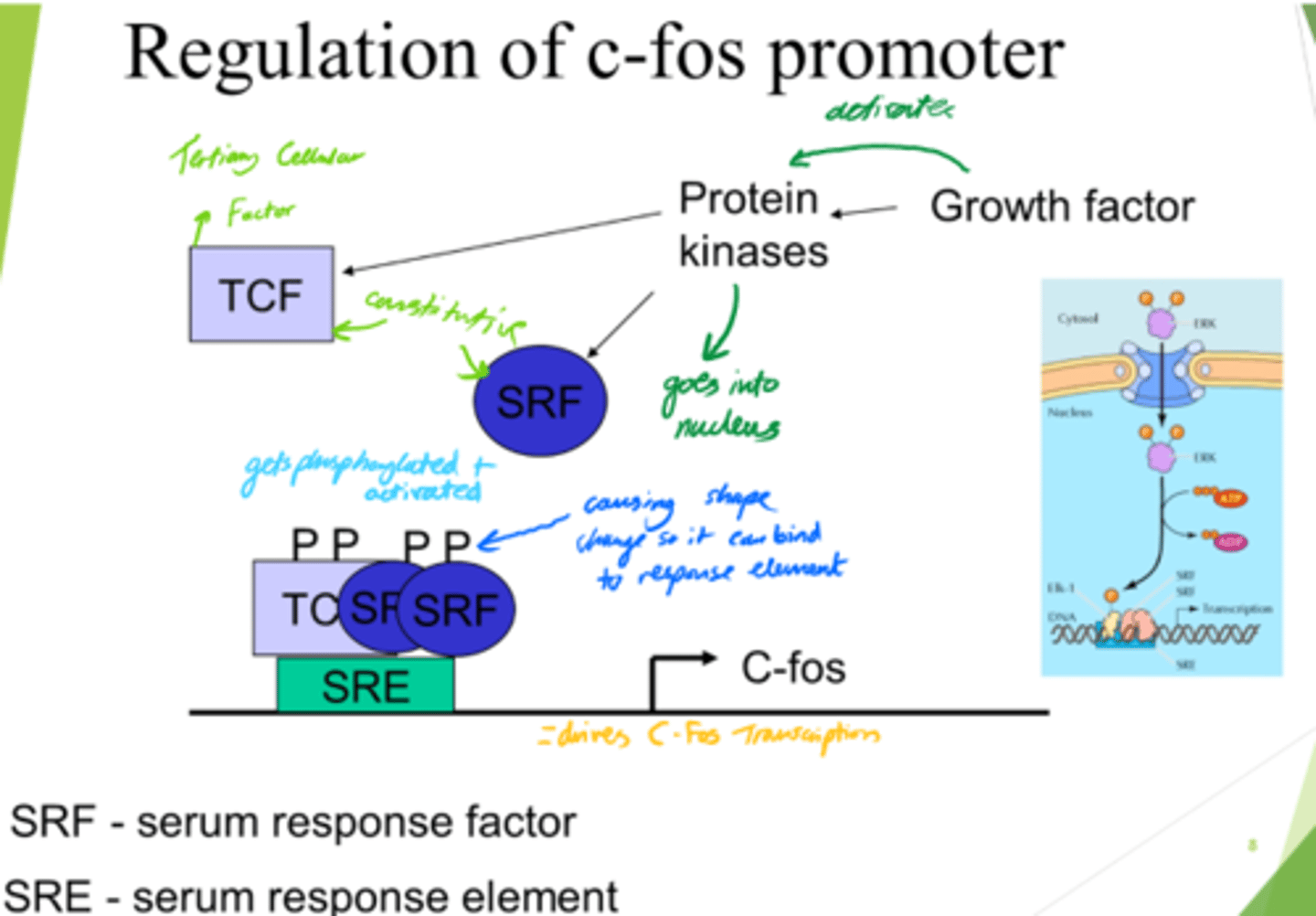
What is TCF?
Ternary Complex Factor
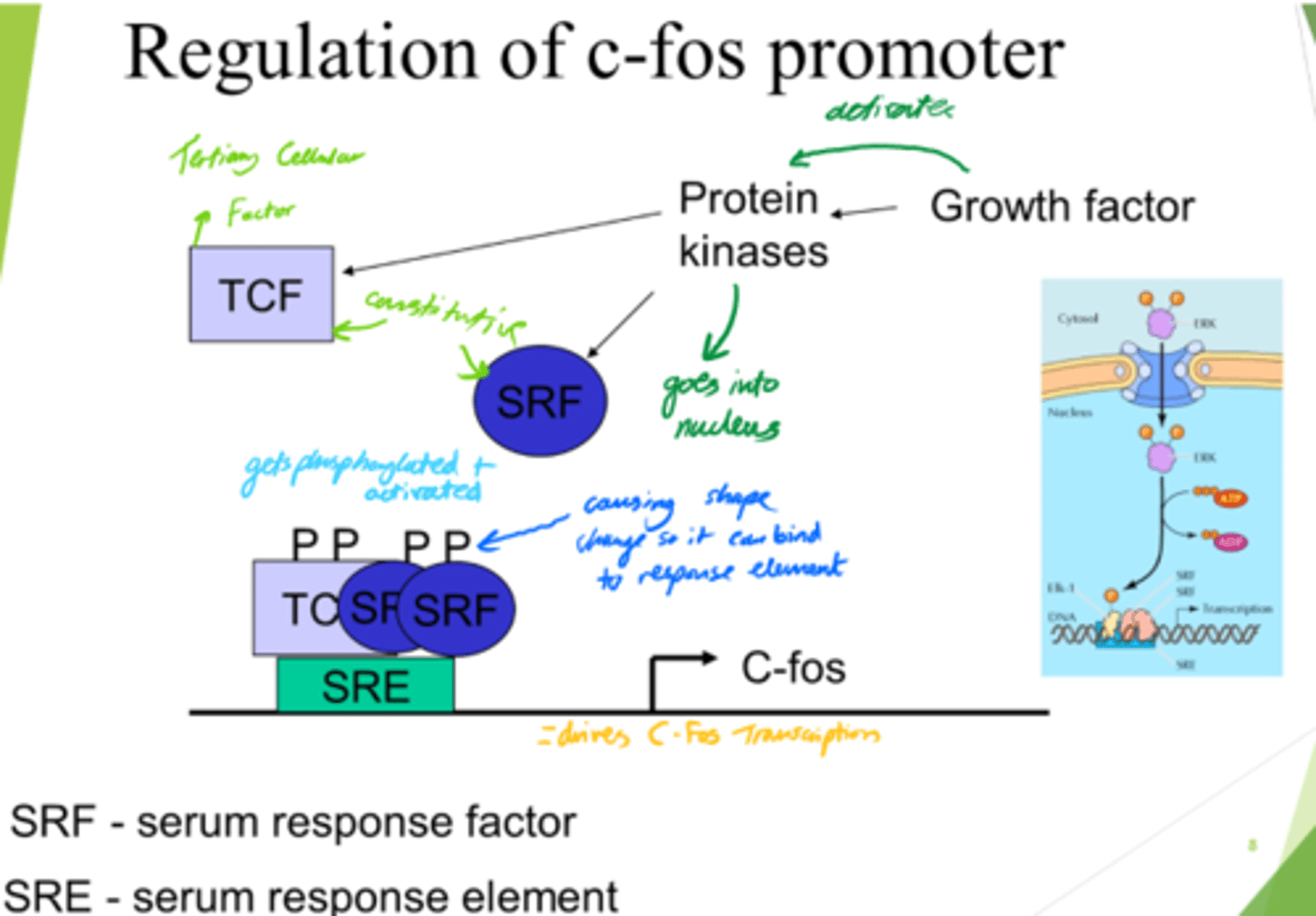
What drives C-Fos transcription, leading to gene expression?
- SRF and TCF bind to SRE on DNA.
- This drives c-Fos transcription.

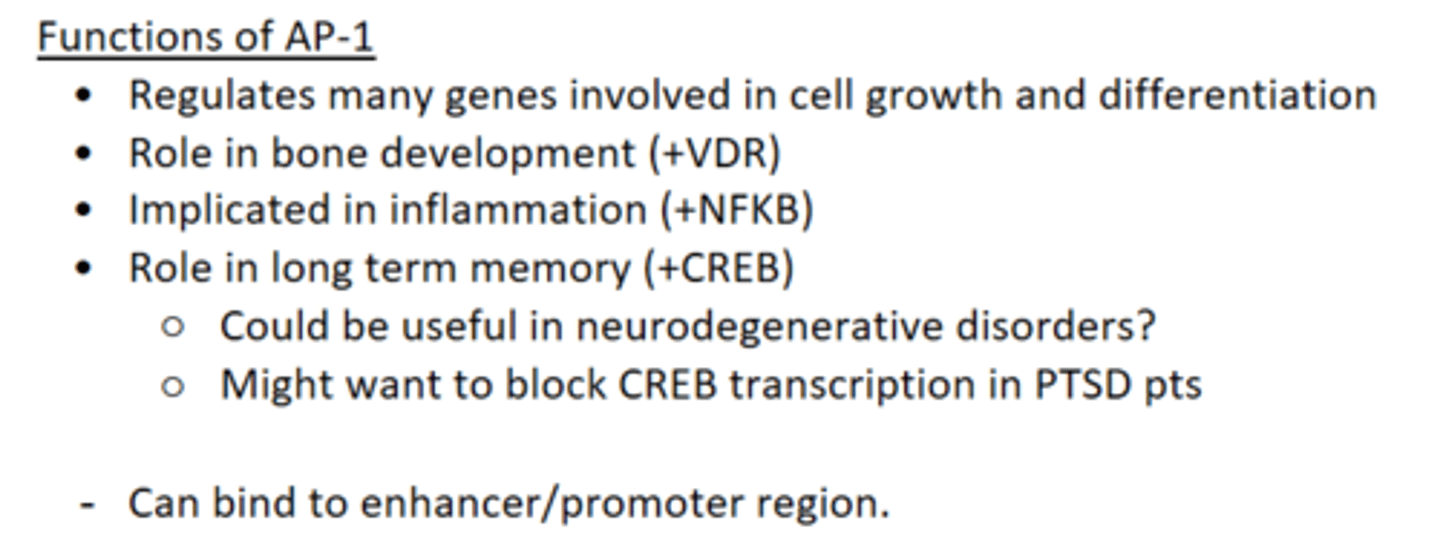
What are the functions of AP-1?
- Regulates genes involved in cell growth and differentiation.
- Role in bone development.
- Role in inflammation (NFKB).
- Role in long term memory.
How do cells proliferate?
- Growth factors bind to receptors.
- Activate intracellular signalling cascade.
- Leads to gene transcription.
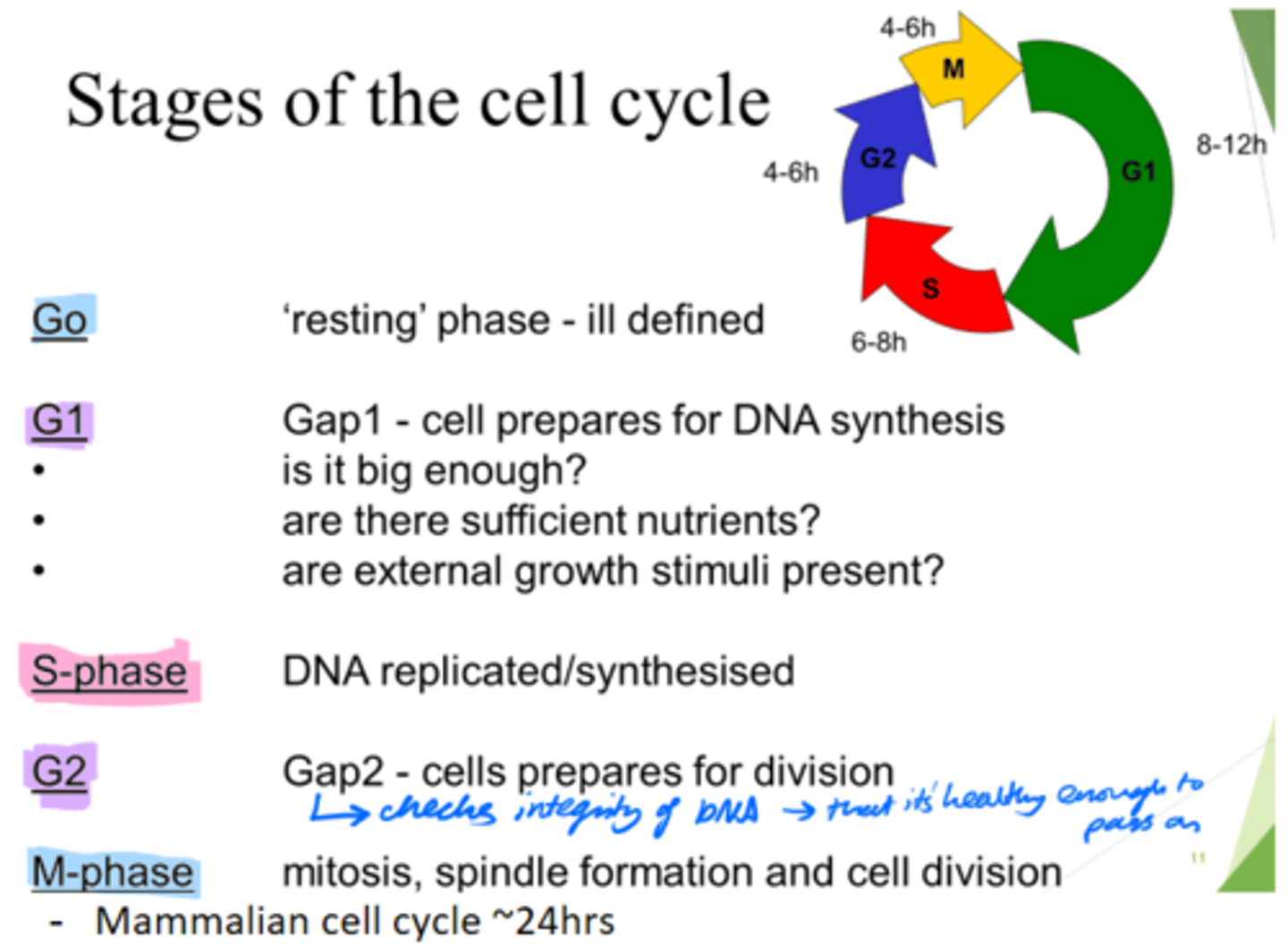
How long does the mammalian cell cycle take?
~24hours.
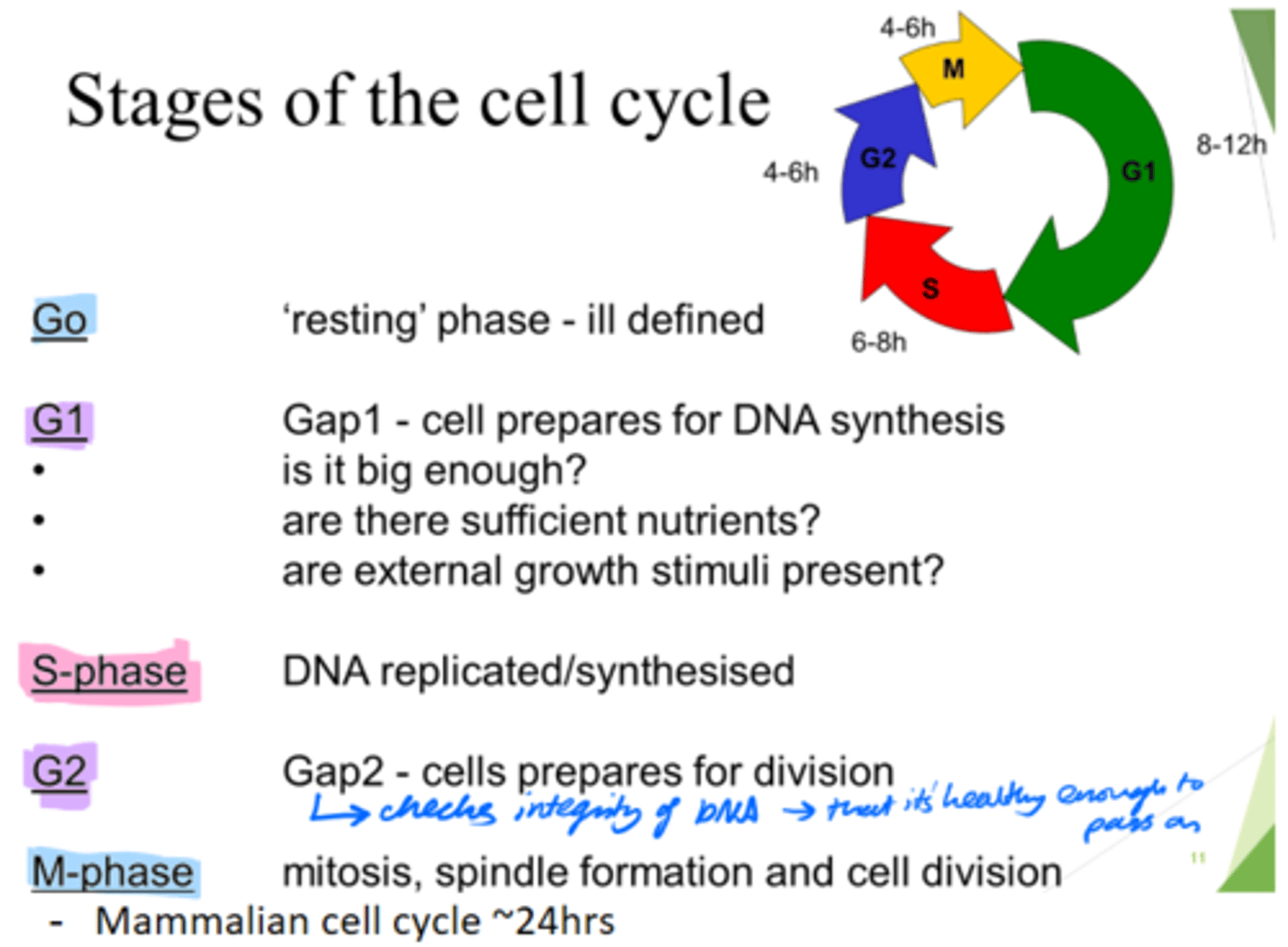
What are the 5 stages of the cell cycle?
Go, G1, S, G2, M-phase
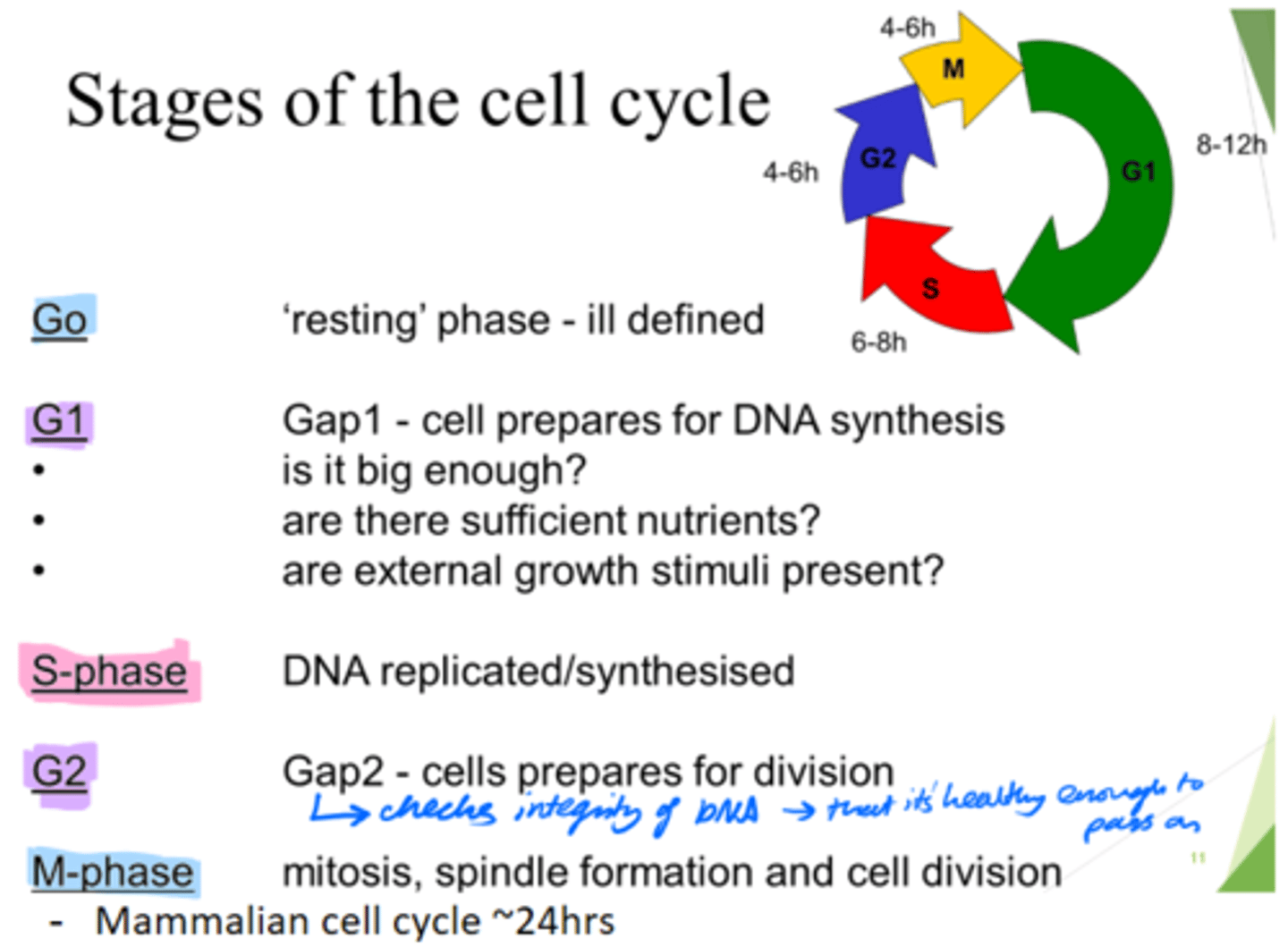
What happens in the Go phase of the cell cycle?
Resting phase

What happens in the G1 phase of the cell cycle?
Gap 1 - cell growth.
Cell prepares for DNA synthesis.
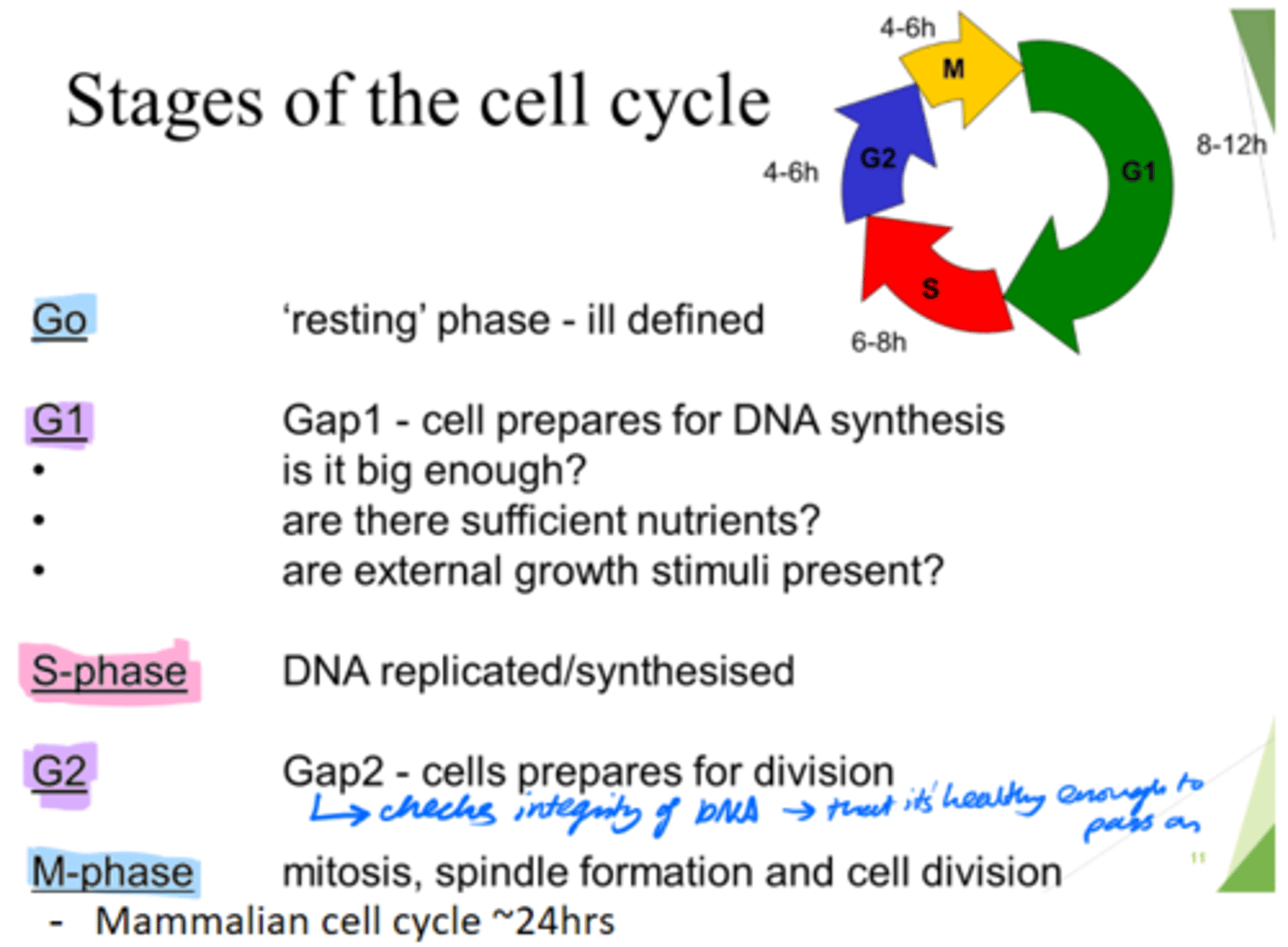
What happens in the S phase of the cell cycle?
DNA is replicated/synthesised.
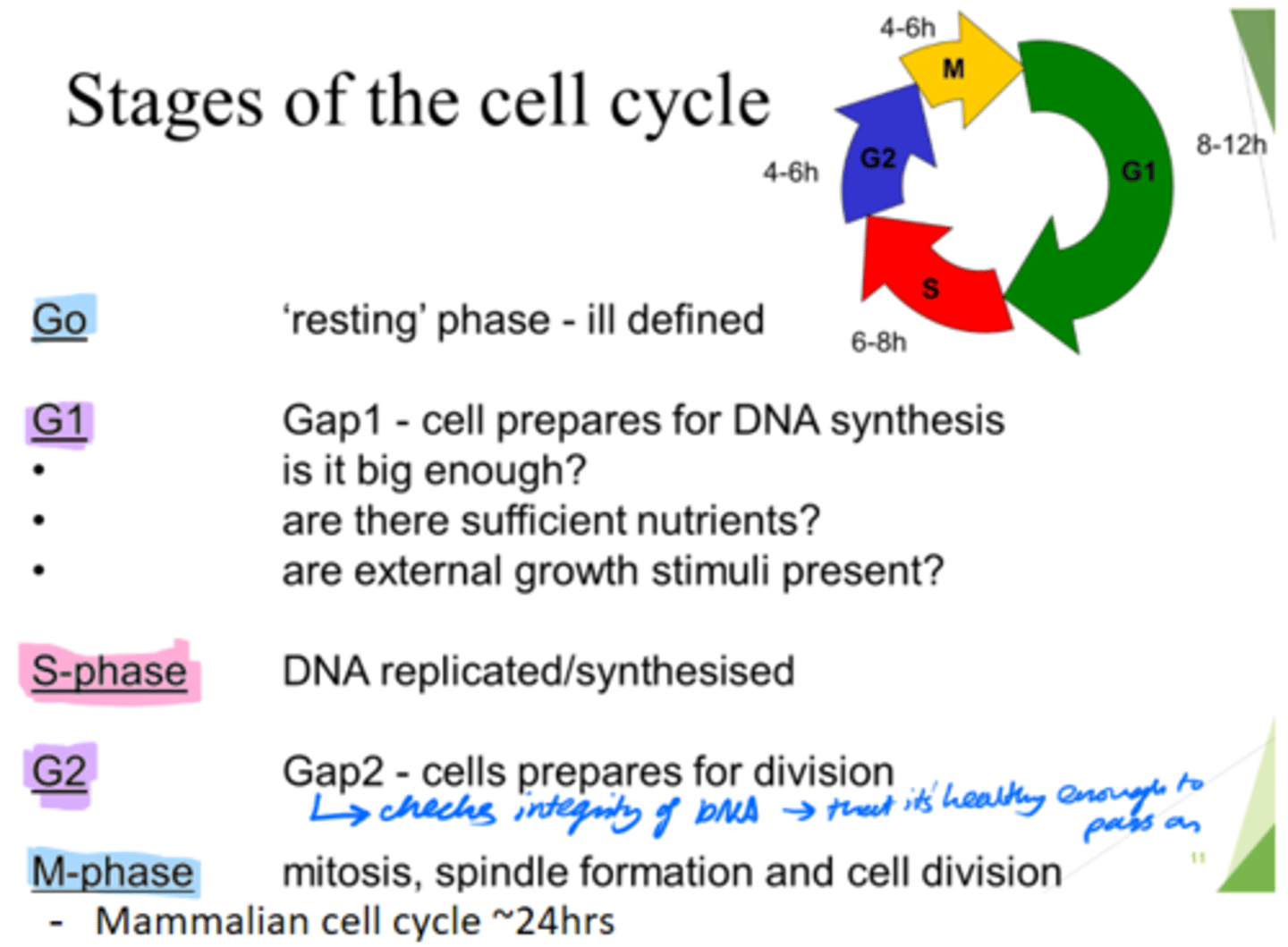
What happens in the G2 phase of the cell cycle?
Gap 2 - cells prepare for division.
- Check DNA integrity; so it's healthy enough to pass on.

What happens in the M phase of the cell cycle?
Mitosis, spindle formation and cell division
What are the 2x protein families involved in driving the cell cycle?
Cyclins, and Cyclin-dependent kinases.
What are cyclins dependent on?
Transcription
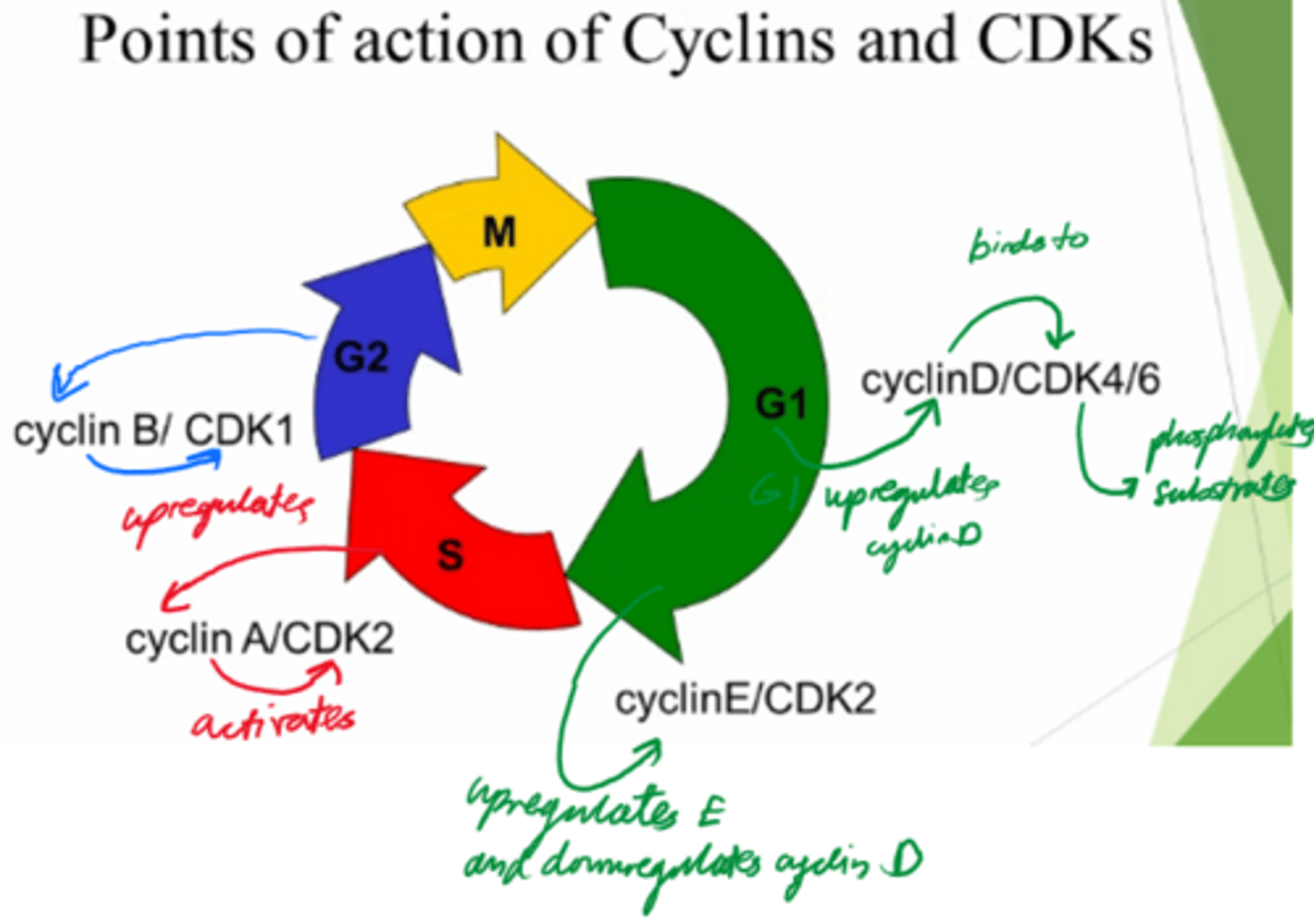
What do cyclins do?
Activate cyclin dependent kinases to control cell cycle.
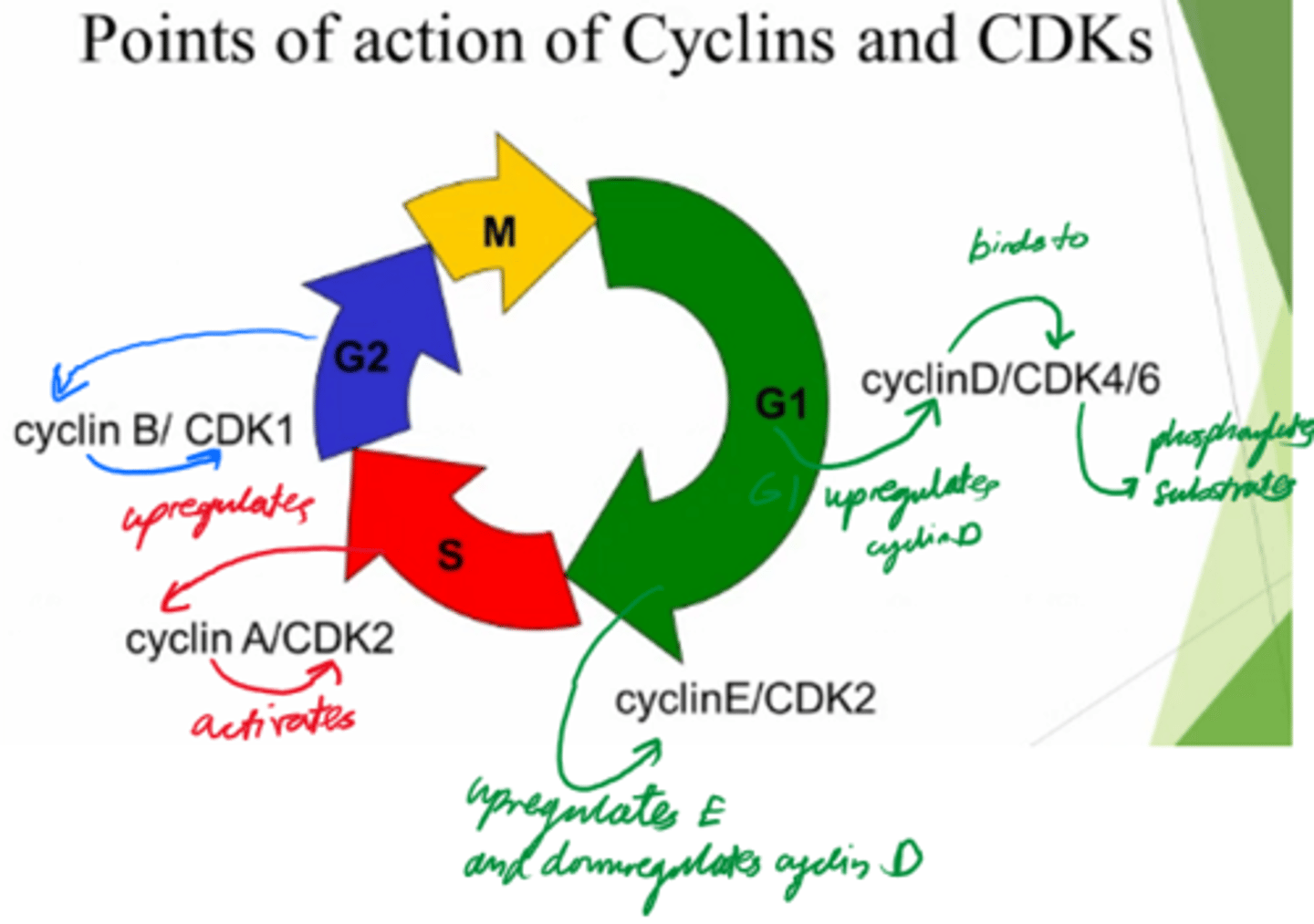
What are Cyclin-dependent kinases dependent on?
Activation by cyclin!

What do Cyclin-dependent kinases do?
- Phosphorylate substrates and drive the cells from the cell cycle.
Control cell cycle!
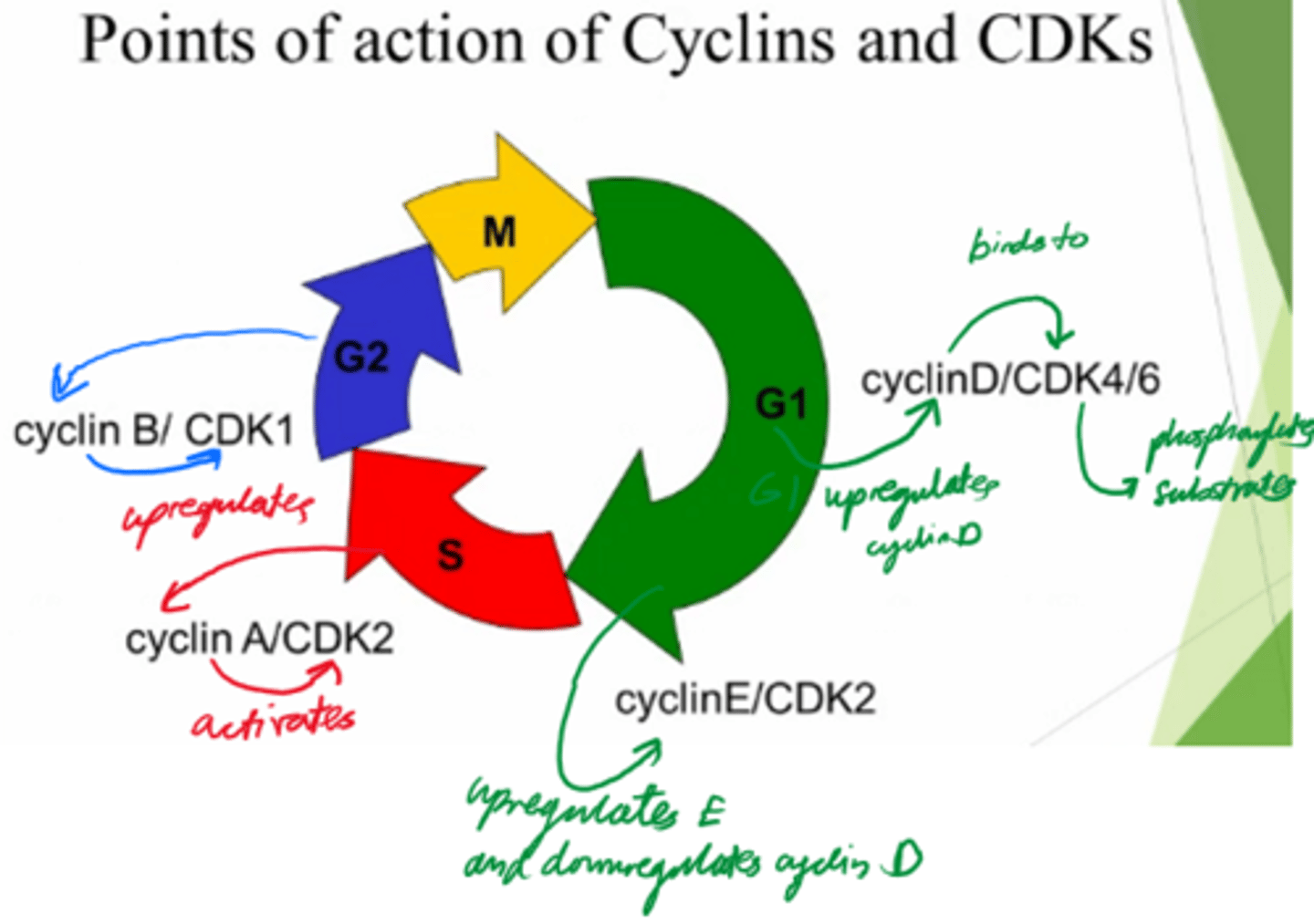
In what phase do Cyclin D and E act?
G1
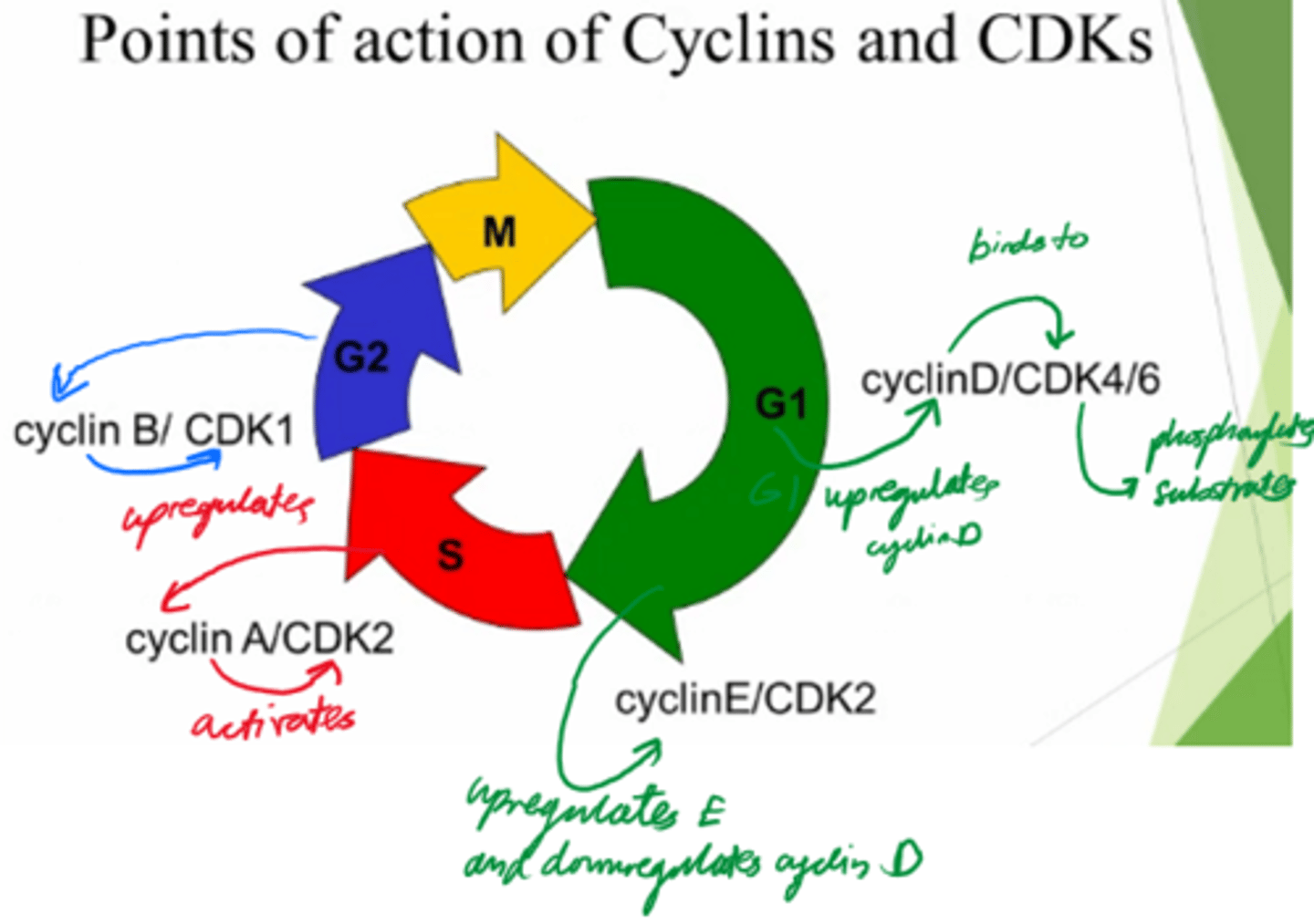
In what phase does cyclin A act?
S
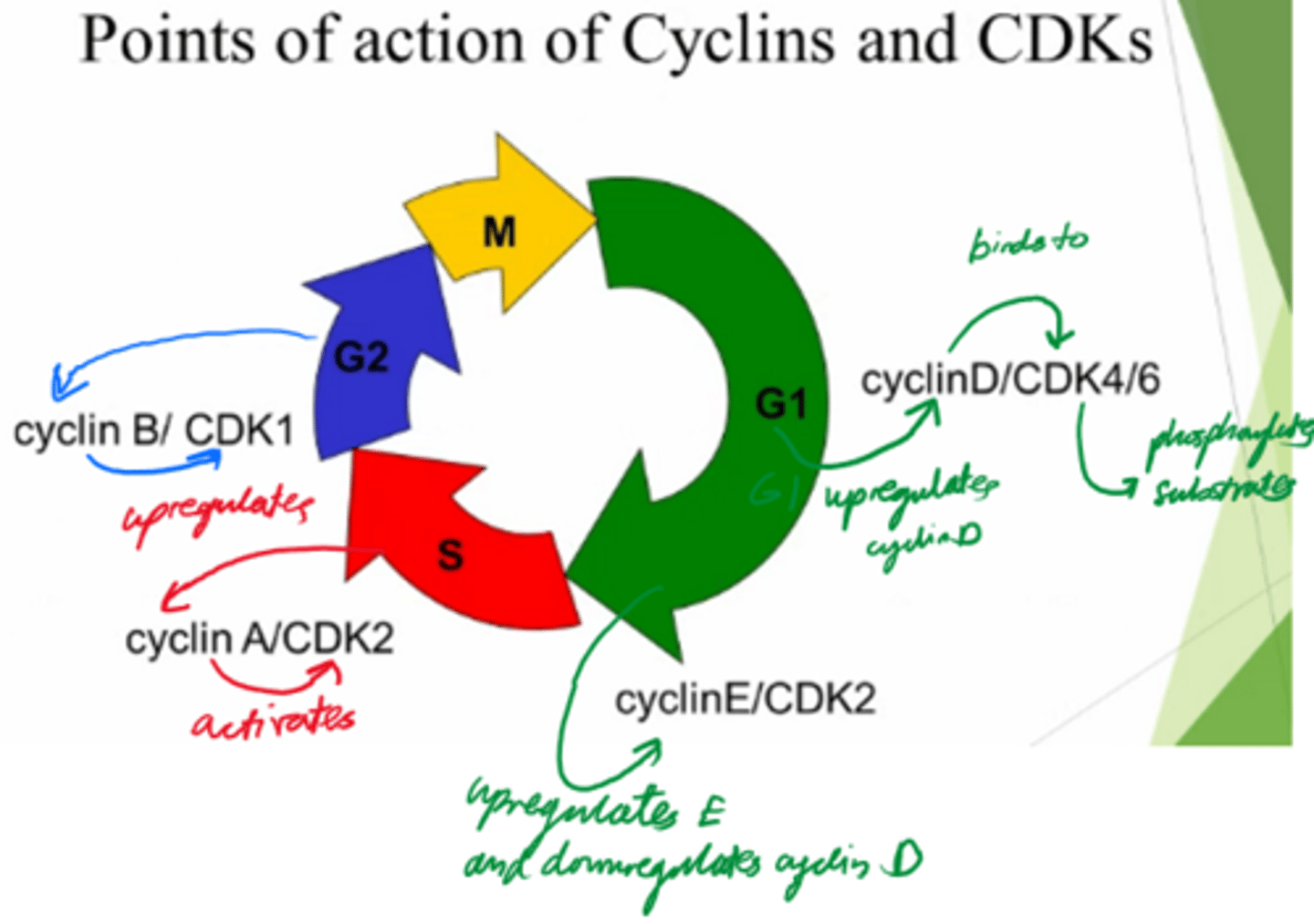
In what phase does cyclin B act?
G2

What happens when growth factors are stimulated in the cell cycle?
- GFs activate kinases.
- Transcription of C-fos and C-jun, promoting cyclin D expression.
What transcription factor is formed by c-fos and c-jun?
AP-1
(binds to promoter region of cyclin D to increase its transcription).
What is pRb?
Retinoblastoma protein
What happens when pRb is phosphorylated?
It releases E2F.
What is the role of E2F in the cell cycle?
Activates cyclin E gene transcription and makes G1 -> S transition irreversible
What is the consequence of dysregulated pRb?
Uncontrolled E2F activation, resulting in unregulated cell cycle progression and cancer.
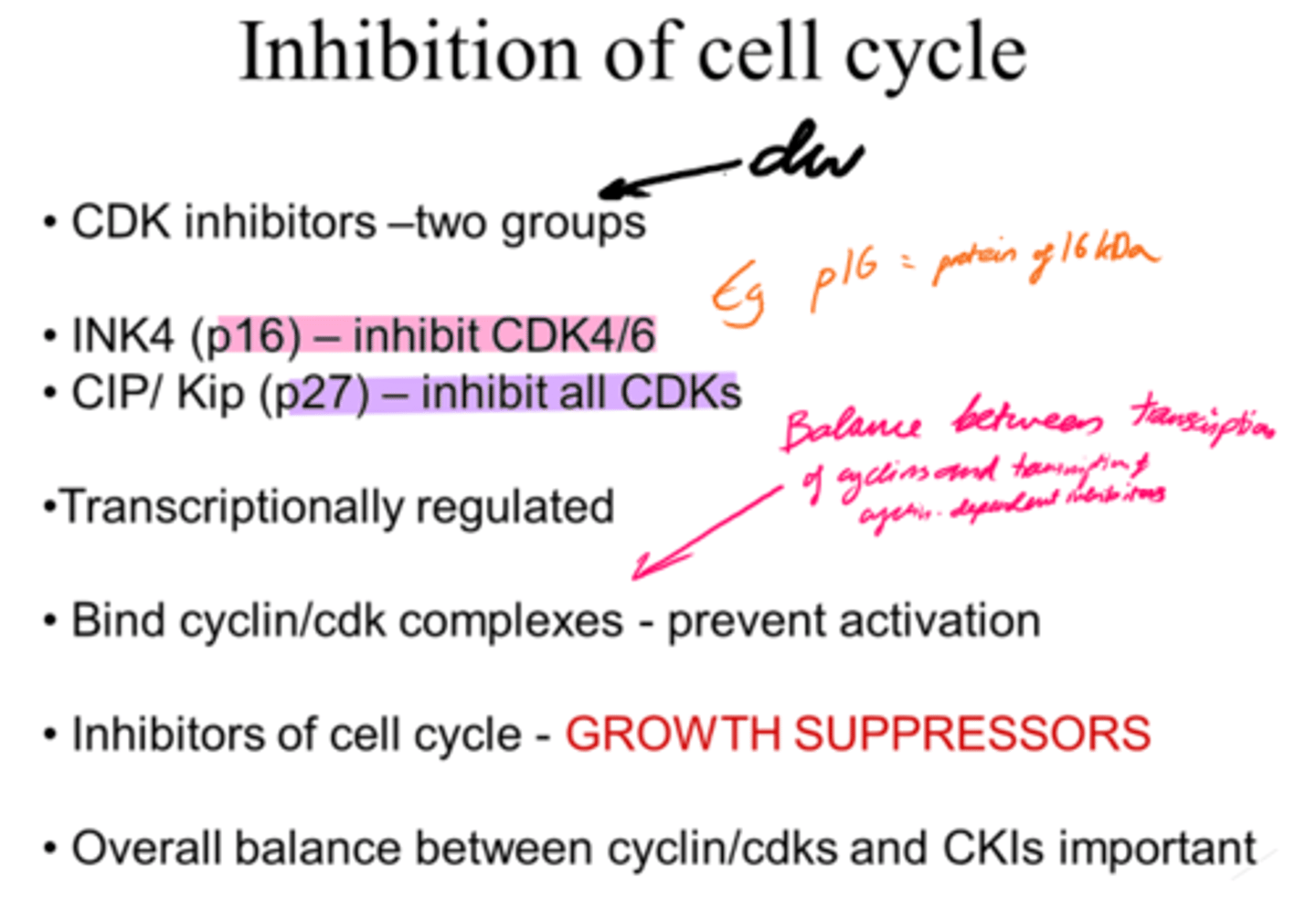
How is inhibition of the cell cycle regulated?
- By Cyclin-dependent kinase inhibitors that bind cyclin/ckd complexes.
- Growth suppressors.
- Transcriptionally regulated.
What happens if DNA integrity is found to be damaged in G2 phase?
cdk inhibitors upregualted to block cell growth.
- DNA then repaired.
- Cyclins upregualted again.
What happens if there's a mutation in EGFR?
If it's made constit active, will signal for growth constantly in absence of growth factor

What happens if there's a mutation in Ras?
Becomes constitutively active

What happens if there is a mutation in pRb?
pRb is a tumour suppressor - loss of function means tumour may not be suppressed.
- If no pRb protein, E2F is free all the time (pRb holds it inactive).
- So it can drive gene transcription/cell cycle.
- If no pRb, nothing to regulate the end of G1.

What is p53 responsible for?
Upregulating cdks by transcription

What happens if there is a mutation in p53?
Loss of funtion means we can't stop the cell cycle or repair DNA.
Damaged DNA could be passed on to daughter cells.

What happens if there's a mutation in p16?
Loss of function means you can't stop cell cycle or repair DNA

Describe the steps of cell growth.
1) Growth Factor activation.
2) MAPK path and Transcription Activation.
3) Cell cycle progression.
4) Regulation by p53.
5) Final cell cycle control.
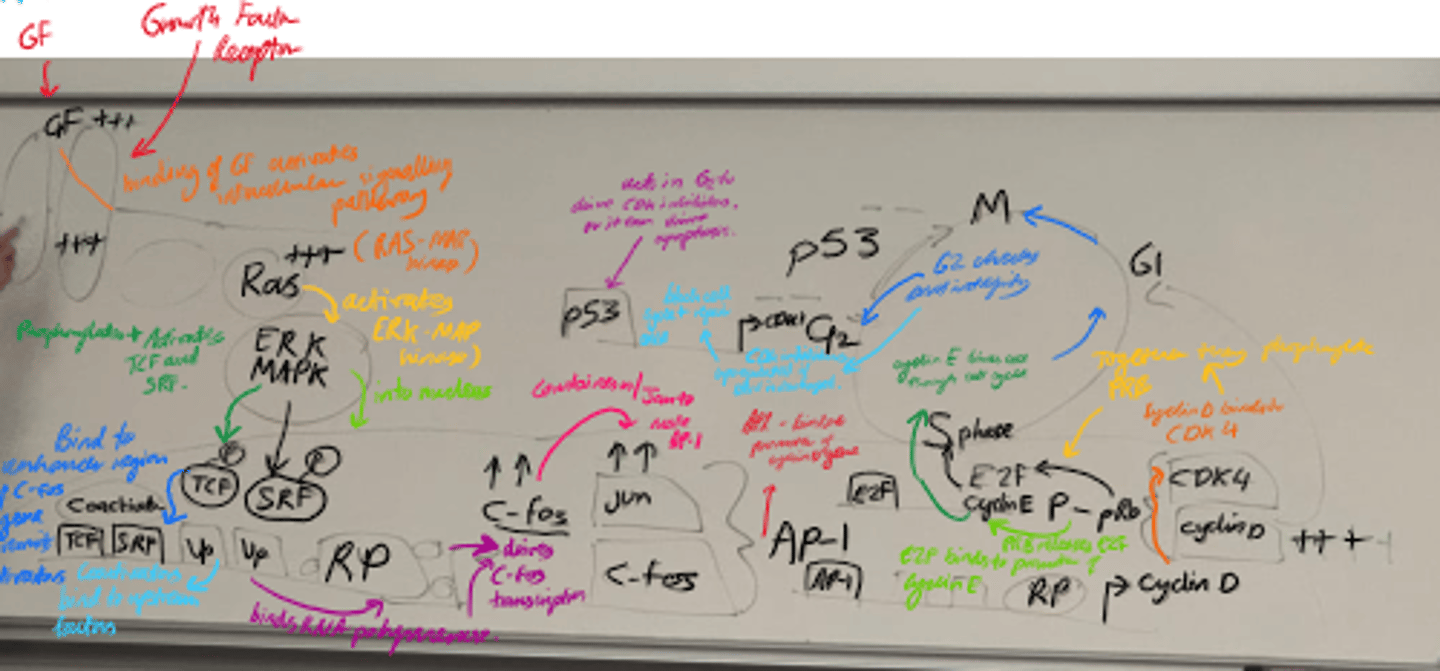
What happens in the Ras-MAPK pathway? (step 2)?
- ERK/MAPK is activated and enters the nucleus.
- It phosphorylates transcription factors eg TCF and SRF, leading to C-Fos transcription.
- C-fos + c-jun = AP-1, which promotes cyclin D transcription.
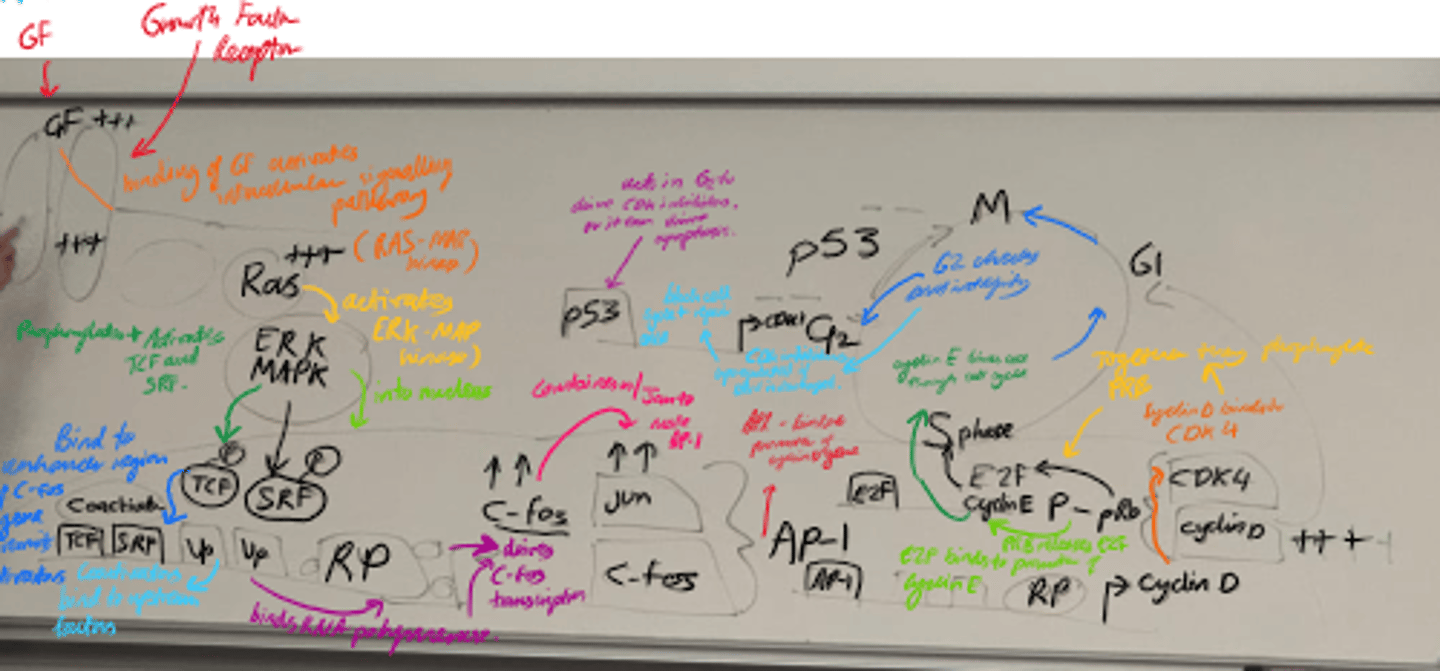
What happens in Cell Cycle Progression (3)?
- Cyclin D binds to CDK4 and forms a complex.
- Complex phosphorylates pRb.
- Phosphorylated pRb releases E2F, allowing cyclin E transcription.
- Drives G1 -> S transition.
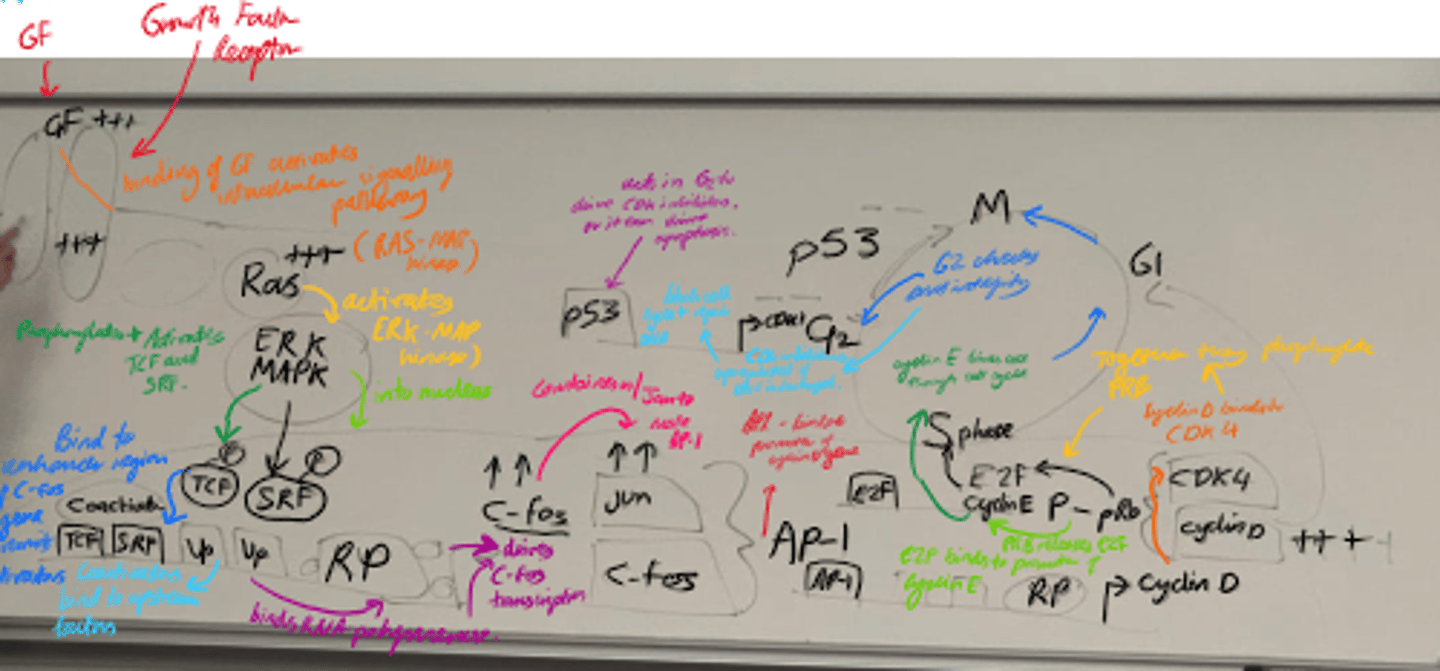
What is the role of p53 in the cell cycle?
- Checkpoint protein that halts cell cycle in G1 if DNA is damaged.
- Induces apoptosis if damage is severe.
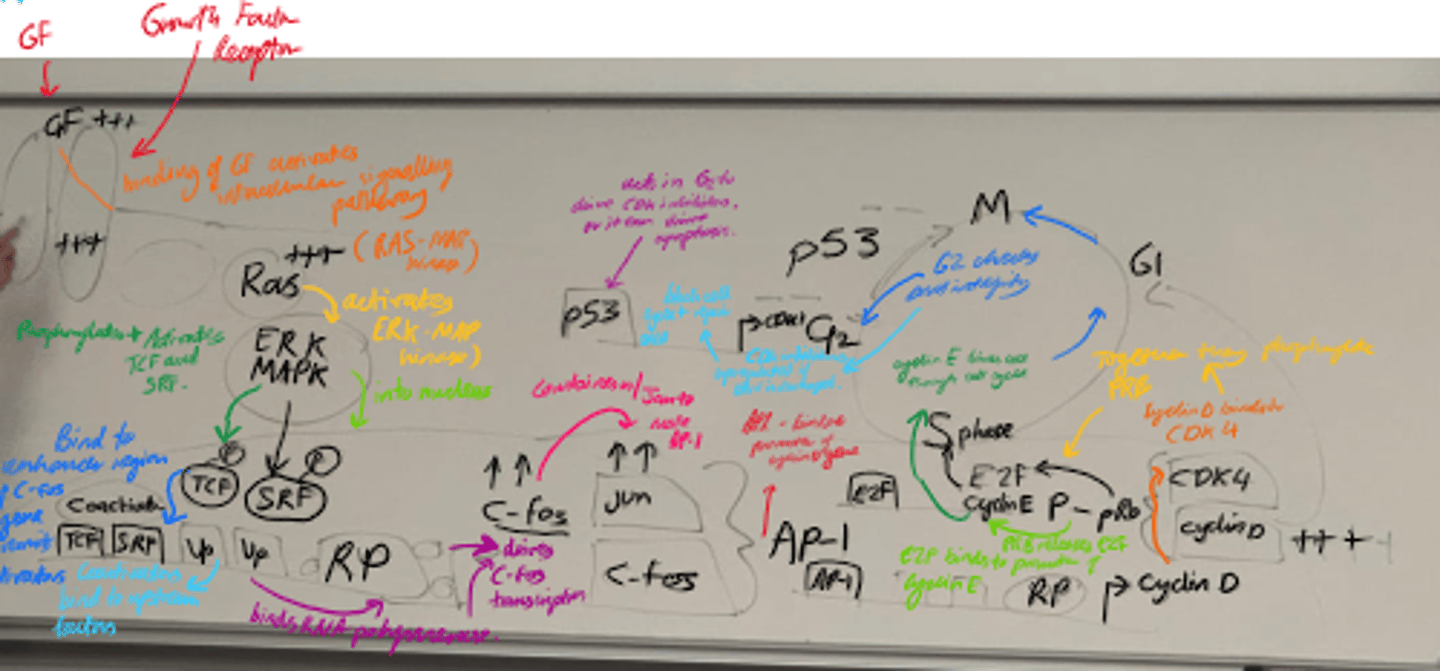
What does cyclin E/cdk2 activity promote?
S-phase entry
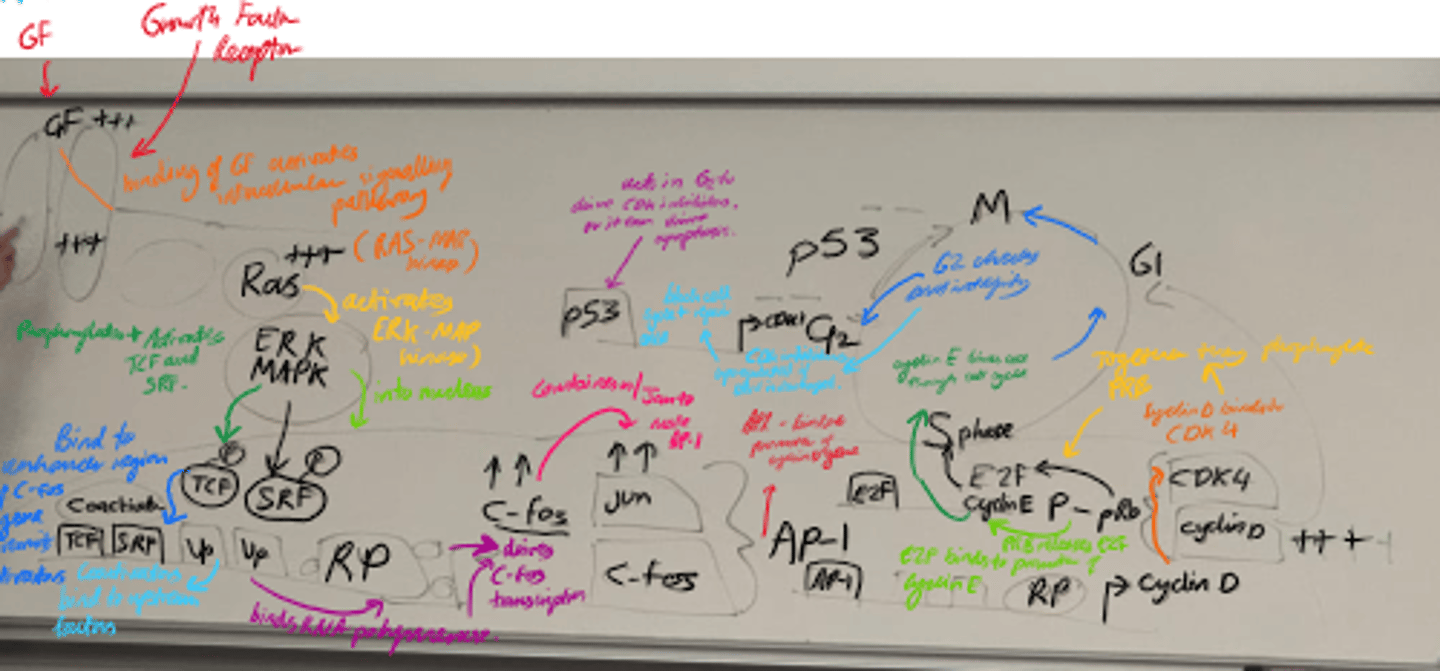
What does Cyclin B/cdk1 control?
G2 -> M transition.
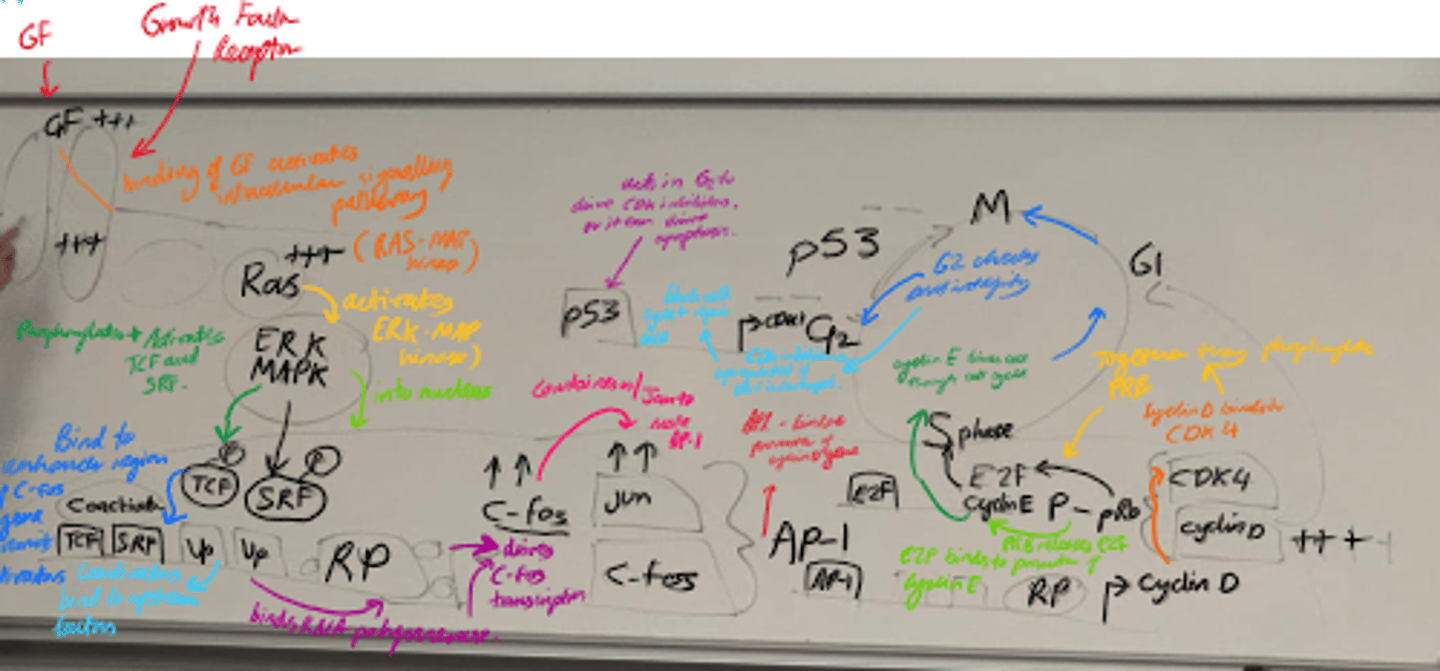
Name some stages in the growth factor signalling pathway that can become mutated.
- Receptors/Ras can become constitutively active.
- Transcription factor can be overexpressed.
- Loss of function of p53/CDKis
How can we control cell proliferation?
- EGFR inhibitors.
- Ras inhibitors.
- HDAC inhibitors.
- p53 replacement.
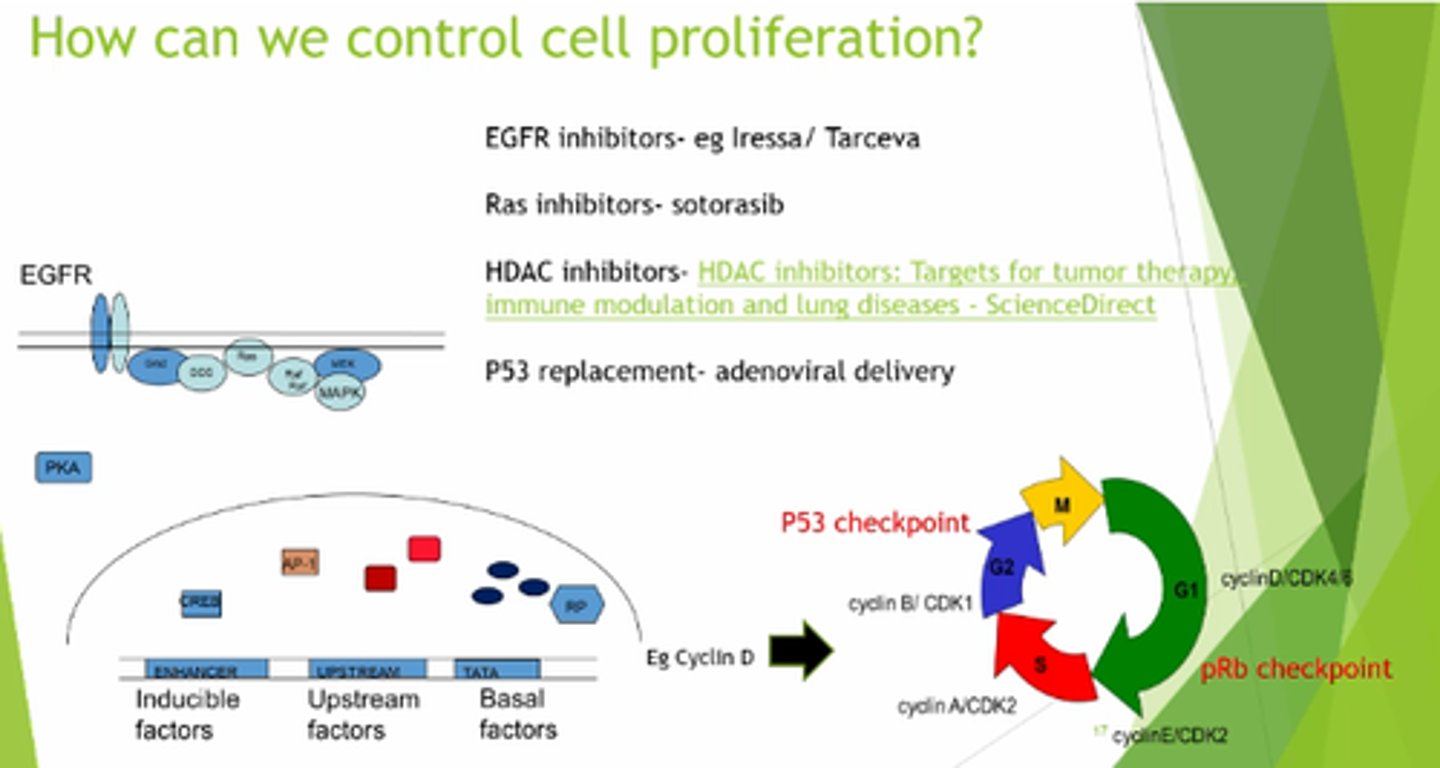
What do HDACs (Histone Deacetylases) do?
Remove acetyl groups to rewind the DNA tightly around histones, reducing availability for transcription.
What is the role of Histone Acetyl Transferase (HAT) in gene expression?
HAT adds acetyl groups to histones, loosening DNA and making it more accessible for transcription.
How does excessive HDAC activity affect tumour suppressor genes?
Blocks TSGs, preventing expression, preventing expression of proteins eg p53 and CDKi's.
How can blocking HDACs help in cancer treatment?
- Allows HATs to stay active.
- Increasing transcription of TSGs.
- Helps regulate cell cycle and prevent cancer.