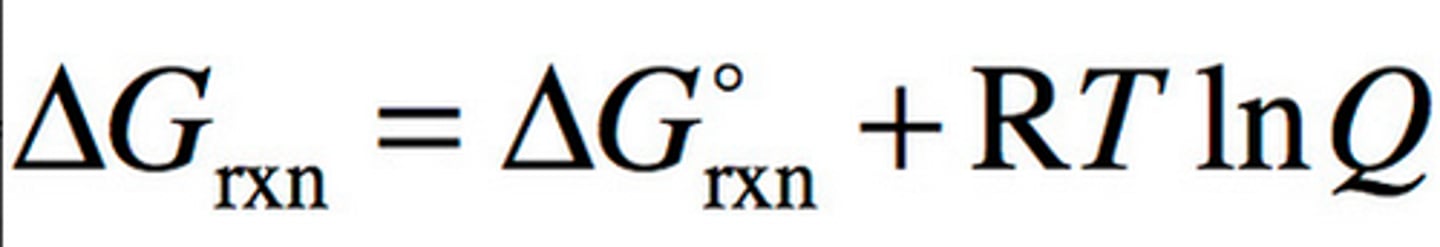Free Energy and Thermodynamics
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
thermodynamics
predict whether a reaction can occur
entropy (S)
random dispersal of energy
spontaneous process
random dispersal of energy
non-spontaneous process
require energy input
microstates
places to occupy
macrostates
contain microstates
Entropy at Phase change
S = q/T
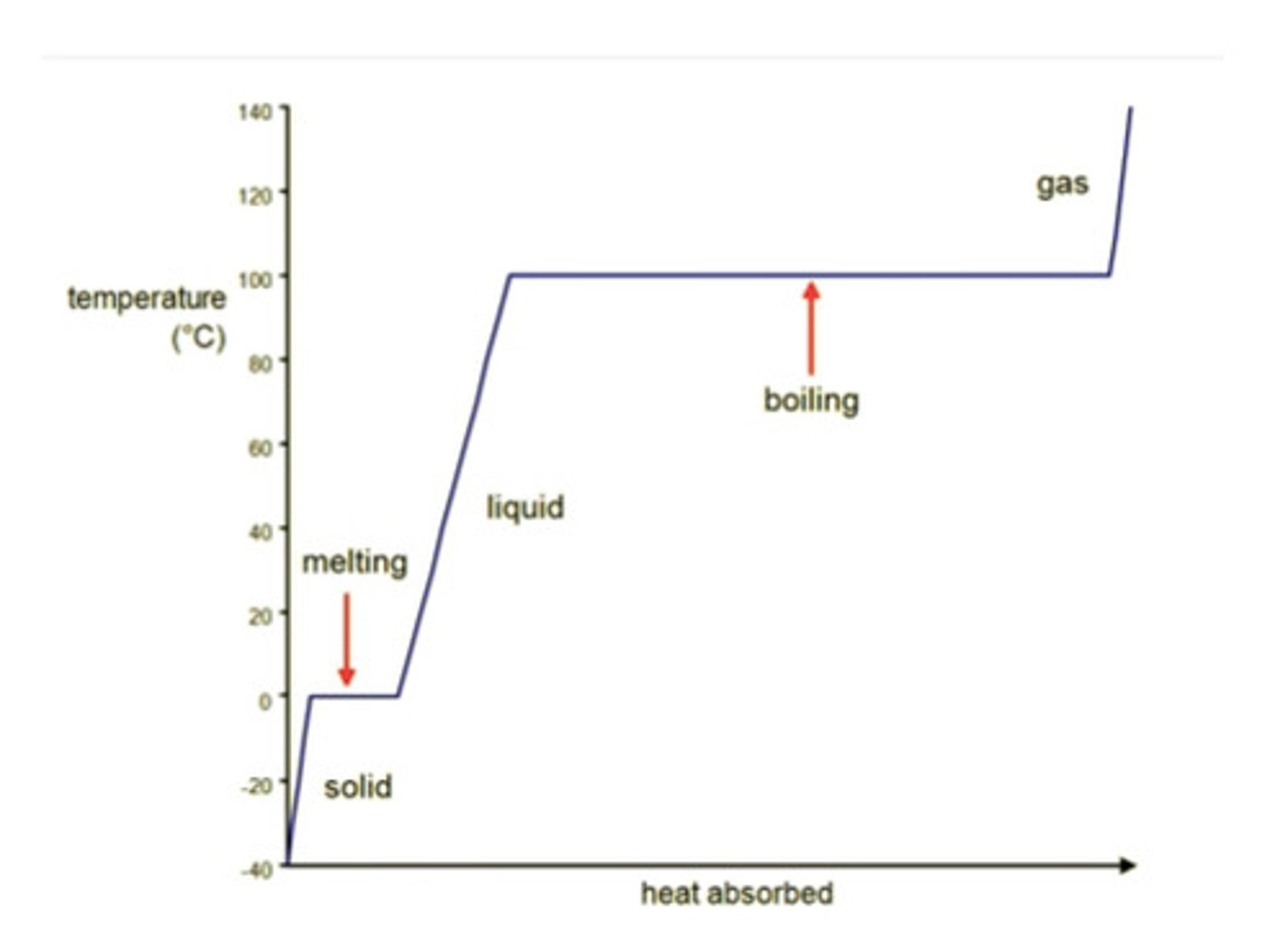
Increase in entropy
larger molecules
smaller ions
endothermic
more product gas molecules
phase change (up)
decrease pressure
Change in Entropy of System
Ssystem = Sreaction = Sproduct - Sreactant
Entropy of Surroundings
Ssuroundings = - Hsystem / T
Entropy of Universe
Ssurrounding + Ssystem
Third Law : Absolute Energy
amount of energy the substance has due to the dispersal of energy through it particles
Gibbs Free Energy
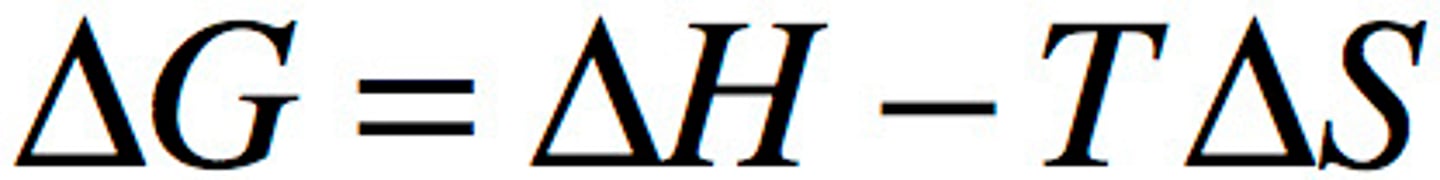
Free Energy of system
Gsys = Hsys - TSsys
Gibbs Free Energy of System
G = Gproduct - Greactant
Gibbs Free Energy and Keq
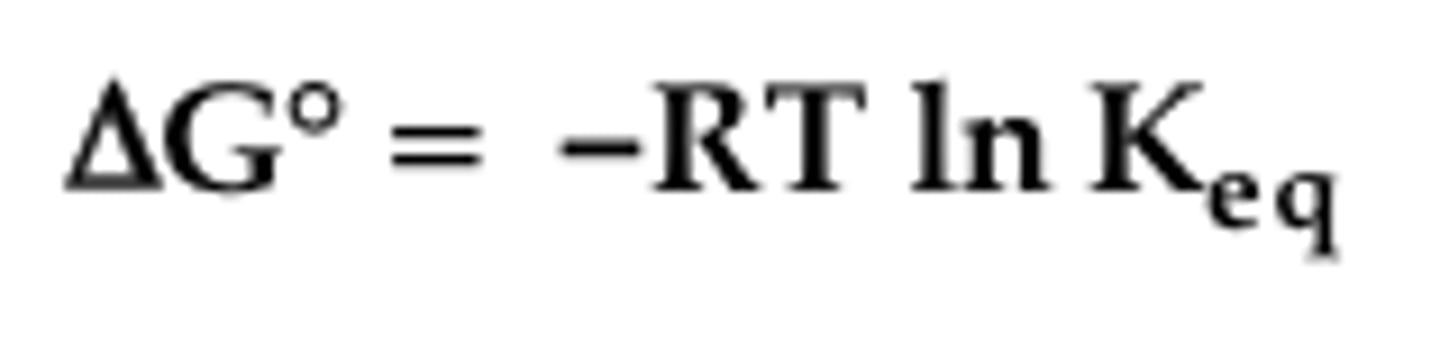
Gibbs Free Energy and Q