Developmental Biology Exam 1
1/141
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
142 Terms
What forms when sperm and egg fuse?
Zygote
What is differentiation?
One cell to many specific cell types
What does morphogenesis refer to?
Generation of shape and form
Which process ensures temporal and spatial ordering of cells?
Patterning
Who demonstrated that development is directed by the nucleus?
Theodor Boveri
What problem did embryologists face with genetics?
Genomic equivalence (all somatic cells have the same DNA)
What mutation replaces antenna with legs in flies?
Antennapedia
Who linked eye color and sex traits to the X chromosome?
Thomas Hunt Morgan
What did Waddington use to study development?
Mutant flies
Which concept says all somatic cells share the same genome?
Genomic equivalence
Differential Gene Expression
The expression of different sets of genes by cells with the same genome.
"central dogma" of biology
DNA -> RNA -> Protein
The promoter
specific region of a gene where RNA polymerase can bind and begin transcription
Exons
Coding segments of eukaryotic DNA.
Introns
Noncoding segments of nucleic acid that lie between coding sequences.
Enhancers
A DNA sequence that recognizes certain transcription factors that can stimulate transcription of nearby genes.
What is recruited to the enhancer/promoter?
Transcription factors
How are enhancers modular?
Each enhancer controls a specific aspect of a gene's expression—for example, driving expression in one tissue, at one time, or under one condition.
Think of enhancers like light switches: a gene might have multiple enhancers, and each one turns the gene "on" in a particular place or developmental stage.
What controls Pax6 expression in different tissues like pancreas, eye, and nervous system?
Modular enhancers for the pancreas, eye and nervous system.
What do binding sites in the Pax6 pancreas enhancer do?
Bind transcription factors (like Pbx1 and Meis) to drive pancreas-specific expression
Histone
protein molecule around which DNA is tightly coiled in chromatin
What are two key chromatin modifications that regulate transcription?
Histone acetylation and DNA methylation
How does histone acetylation affect transcription?
It activates transcription by loosening chromatin
How does DNA methylation typically affect transcription?
It inhibits transcription (methylated promoters are silent)
How does DNA methylation inhibit transcription?
By preventing transcription factor binding and recruiting histone deacetylases
What happens to hemoglobin gene expression during development?
ε-globin (embryo) → γ-globin (fetal) → β-globin (adult)
Why do globin genes switch during development?
Promoter methylation silences one gene and activates another
What is alternative RNA splicing?
One pre-mRNA can be spliced into different mRNAs that encode different proteins
What is an embryonic example of alternative RNA splicing?
Type II procollagen splice variants in chondrocytes
How can translation be regulated by microRNAs (miRNAs)?
miRNAs block ribosome assembly and prevent translation
What protein complex do miRNAs use to silence mRNAs?
RISC (RNA-induced Silencing Complex)
What is post-translation regulation?
Protein folding and modification after translation
What are three ways mRNAs can be regionally localized in cells?
Anchoring to a specific region, localized protection, active transport
What is a ligand in cell signaling?
A signaling factor that binds a receptor
What is induction in developmental biology?
One cell population influencing nearby cells at close range
During eye development, what acts as the inducer for lens formation?
The optic vesicle (extension of the developing brain)
Why can't all tissues act as inducers?
They lack expression of important genes needed for induction
What does it mean for a tissue to be competent?
It can respond to an inducing signal
What is reciprocal induction?
An induced tissue later induces other tissues, including the original inducer
Which gene is required for vertebrate eye development?
pax6
In which tissue is Pax6 required for lens induction?
Surface ectoderm
What paracrine signals from the optic vesicle induce gene expression in surface ectoderm?
Fgfs and BMPs
Which genes are induced in lens ectoderm by signals from the optic vesicle?
otx2, pax6, sox3, maf
What is the extracellular signal in the Hedgehog pathway?
Hedgehog protein
What is the receptor for Hedgehog?
Patched
If Hedgehog is not present, what happens to transcription?
Gli Patched (Ptc) inhibits Smoothened (Smo) -Smo is degraded Gli is tethered to the microtubules and is cleaved into a transcriptional repressor
If Hedgehog is present, what happens to Gli?
Hedgehog (Hh) binds to Patched (Ptc) and prevents it from inhibiting
Smoothened (Smo). Smoothened releases Gli from the microtubules as Gli then acts as a transcriptional activator.
What is the extracellular signal in the Wnt pathway?
Wnt protein
What is the receptor for Wnt?
Frizzled
If Wnt is not present, what happens to β-catenin?
Glycogen Synthase Kinase 3 (GSK3) inhibits b-catenin (b-cat) by promoting its
degradation. b-catenin is a transcription factor that is degraded in the absence of Wnt.
If Wnt is present, what happens to β-catenin?
Wnt binds to Frizzled, which binds to Disheveled.
Disheveled pulls GSK3 away from b-catenin.
b-catenin is not degraded, but accumulates,
and enters the nucleus to turn on transcription.
If a gene is active, what else will be present?
Its mRNA and protein (maybe) will be present
Ways to Assay Gene Expression
• In situ hybridization (1 mRNA)
• RNA-Seq (whole transcriptome)
• Immunohistochemistry (proteins)
In Situ
In situ is used to assay genes by visualizing specific DNA or RNA sequences directly within cells, tissues, or chromosomes, rather than extracting the nucleic acids from their biological context. This technique, called in situ hybridization (ISH), uses labeled probes that bind to their complementary gene targets. By preserving the cellular morphology, ISH reveals the spatial and temporal patterns of gene expression
In Situ Step #1
Make a probe that is complementary to the mRNA
The probe contains an epitope that will bind an antibody.
The nucleotide sequence of this probe is referred to as
“antisense”
In Situ Step #2
Soak the embryo in the probe solution
The probe binds to the mRNA if the gene is expressed
In Situ Step #3
Soak the embryo in the antibody solution
The antibody binds to the probe if the gene is expressed
What does this antibody have attached to it?
An enzyme that can convert a substrate into a colored product
In Situ Step #4
Soak the embryo in the substrate solution
The substrate is converted into a colored product
RNA-Seq (whole transcriptome)
#1 Purify mRNAs from each sample
#2 Construct a DNA copy of each mRNA
#3 Sequence and measure differential gene expression between tissues
Immunohistochemistry (proteins)
A primary antibody binds specifically to an embryonic protein, a secondary antibody binds to the primary antibody. The secondary antibody is linked to a
fluorescent “tag.” More than one protein can be stained with different colors.
Mutational Analysis
Mutational analysis is a genetic testing technique that identifies specific genetic changes (mutations) within a gene or region of DNA to understand disease, develop therapies, or identify genetic variations
Mutational Analysis Example
Parents are treated with a mutagen
Mutant gene passed from generation to generation
Homozygous mutant embryos will show a developmental phenotype
Mutational Analysis Guidelines
You must screen through a large number of embryos
You could search for phenotypes within a specific tissue
Mutations will be randomly distributed within the genome
Not all model systems allow for mutational analysis (Forward Genetics)
There are non-mutation-based ways of knocking down a gene’s function
(Reverse Genetics).
How can RNA interference (RNAi) can be used to prevent translation?
You can introduce double-stranded RNA for a gene you want to silence.
It will be incorporated into the cell’s RISC complex and used to target
a specific mRNA for degradation.
“Find it, Move it, Lose it”
Is it there?
What happens if we take it away?
What happens if we move it?
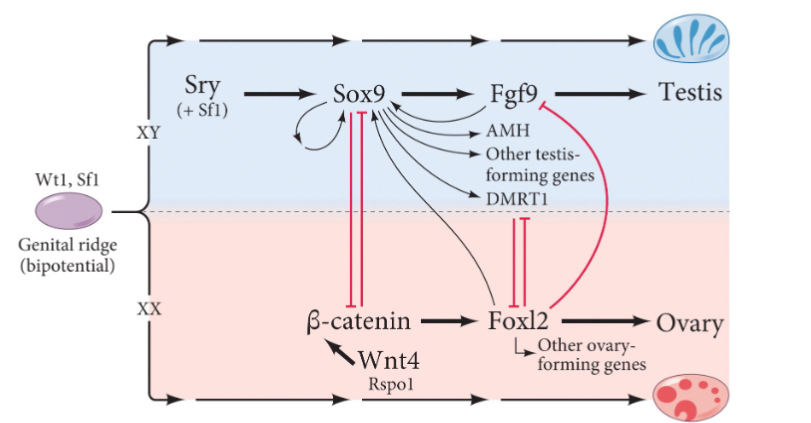
Mammalian Sex Determination is Controlled by what gene?
the SRY. The Sex-determining Region on the Y chromosome
Model for Sex Determination in Mammals
SRY blocks b-catenin signaling and ovary development.
SRY promotes Fgf signaling, through Sox9, and testis development
Without SRY, b-catenin/Wnt signaling promotes ovary development.
If SRY is present, it’s expression is short (a few hours) and is initiated earlier
Gametogenesis
The differentiation of primordial germ cells (PGCs) into
either eggs or sperm
Gametogensis
The differentiation of primordial germ cells (PGCs) into either eggs or sperm.
When is the germ cell lineage separated from the somatic cell?
Germ cell lineage (germ line) is separated
from the somatic cell lineage very early.
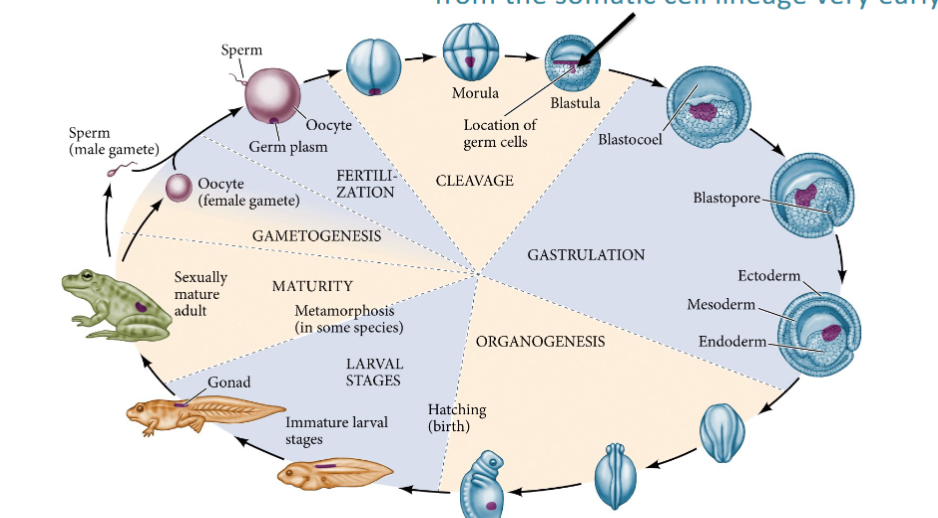
Where do PGCs migrate?
Into the developing gonad. PGCs differentiate into sperm within the testis.
PGCs differentiate into oocytes within the ovaries
Fertilization
1 Movement of gametes toward each other
2. Binding and recognition of gametes
3. Membrane fusion
4. Block to polyspermy
5. Fusion of genetic material
6. Activation of egg metabolism and development
The Mature Sperm Cell
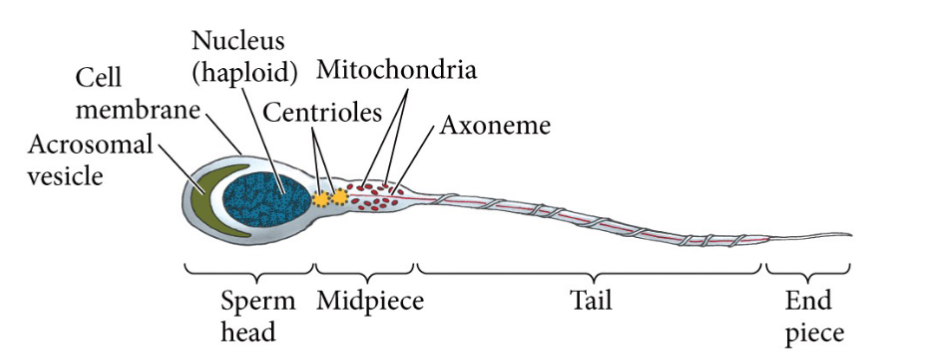
Acrosome
Contains enzymes that digest proteins and carbohydrates that
surround the oocyte
Flagellum
Contains microtubules and dynein motor protein that whip the tail
to propel the sperm cell forward
Midpiece
contains lots of mitochondria.
Fertilization takes place within the _____ of the fallopian tube
Ampulla
Ampulla
Oviduct
Fimbriae
Finger-like projections at the end of
the fallopian tube that promote entrance of the
oocyte into the fallopian tube.
Mammalian oocytes are arrested when?
Metaphase II of Meiosis II at the
time of fertilization.
Factors Driving Sperm Translocation Toward the Oocyte
Sperm flagellum movement
Thermotaxis
-attracted to 2o C warmer ampulla
Chemotaxis
-attracted to progesterone from cumulus
cells surrounding the oocyte.
Against flow of fluid (rheotaxis)
Uterine contraction
What is the outermost protective layer surrounding mammalian eggs?
Cumulus cells
What is the outermost protective layer surrounding sea urchin eggs?
Jelly
What structure surrounds the plasma membrane of sea urchin eggs?
Vitelline membrane
What structure surrounds the plasma membrane of mammalian eggs?
Zona pellucida
What layer is common to both mammalian and sea urchin eggs, lying just beneath external coverings?
Plasma membrane
Oviduct induces sperm maturation through _____
Capacitation
Capacitation
Allbumin and bicarbonate ions
(within the female reproductive tract) ——> Removal of membrane cholesterol
+ Pathway activation =
Greater membrane fluidity
Alters membrane proteins
Increase flagellum movement
How does CatSper mediate sperm hyperactivity?
Progesterone activates CatSper calcium channels (via ABHD2 and other regulators), causing Ca²⁺ influx into the sperm flagellum. This calcium rise stimulates hyperactivated motility, allowing stronger, whip-like tail movements needed for fertilization.
Mammalian sperm cells must...
-pass through the cumulus cell layer
-bind to and recognize the zona pellucida
-bind to and fuse with the plasma membrane
Sperm cells bind to what protein on the Zona Pellucida?
ZP2 (Zona Protein 2)
Acrosome Reaction
The acrosome fuses with the sperm cell membrane
and releases enzymes that degrade the egg’s jelly
in the sea urchin.
The acrosome reaction exposes internal sperm
proteins to the outside (Izumo) so that they can
bind to proteins (Juno) on the surface of the oocyte.
This contributes to sperm-oocyte membrane fusion.
Polyspermy
The entrance of more than one sperm during fertilization
resulting in abnormal chromosome number (aneuploidy)
How many sperm enter the female reproductive system, and how many reach the egg?
About 200–300 million enter, but only a few hundred reach the egg.
What are the two main cellular changes that prevent polyspermy?
The fast block (not found in mammals) and the slow block.
How does the fast block to polyspermy work?
Sperm binding causes Na⁺ to rush in, depolarizing the egg from –70 mV to +20 mV, preventing additional sperm binding.
What experiment demonstrated the importance of Na⁺ in the fast block?
Lowering Na⁺ kept the membrane negative, resulting in a higher % of polyspermic eggs.
What triggers the slow block to polyspermy?
Cortical granules fuse with the plasma membrane and release contents between the plasma membrane and vitelline/ZP.