Chemical bonds retake
0.0(0)
Card Sorting
1/30
There's no tags or description
Looks like no tags are added yet.
Last updated 5:52 AM on 5/13/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
1
New cards

False

2
New cards

True

3
New cards

False

4
New cards

True

5
New cards

False

6
New cards

False
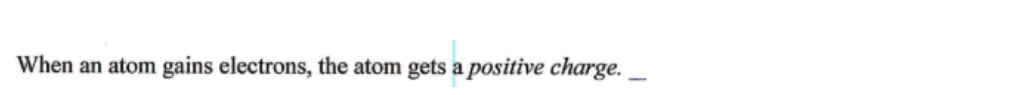
7
New cards

A metallic substance

8
New cards

Is always zero

9
New cards

it gives up electrons

10
New cards

They have low melting points

11
New cards

Polar covalent

12
New cards

a force that holds atoms that are oppositely charged

13
New cards

nonpolar covalent

14
New cards

ions are surrounded by ions of the opposite charge

15
New cards

The ionic compound has a high melting point

16
New cards

Sodium only

17
New cards
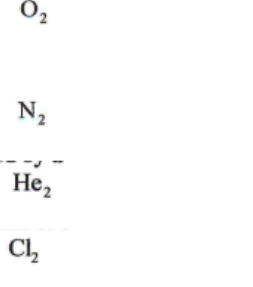
O2

18
New cards

PCl3

19
New cards

They are composed of anions and cations

20
New cards

To obtain noble-gas electron configuration

21
New cards

Cl2

22
New cards

Nitrogen

23
New cards

only when it is melted or dissolved in water

24
New cards

double covalent bond

25
New cards

They have mobile valence electorns

26
New cards

Al2O3

27
New cards
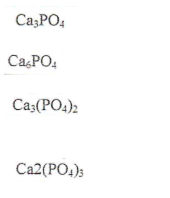
Ca3(PO4)2

28
New cards
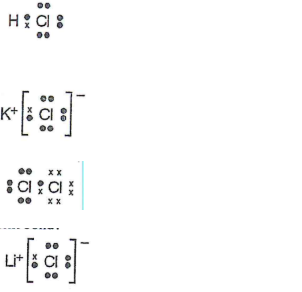
HCl

29
New cards
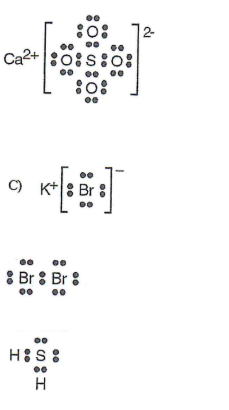
Ca2+

30
New cards
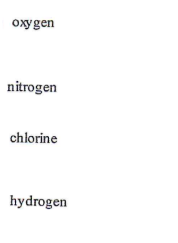
oxygen

31
New cards

the one that is full
