Unit 7 Energetics
1/84
Earn XP
Description and Tags
1.1.1 1.1.2 1.1.3 1.1.4 1.2.1 1.2.2 1.2.3 1.2.4 1.2.5 1.3.1 1.3.2 1.3.3 1.3.4 1.3.5 1.4.1 1.4.2 1.4.3 1.4.4
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
Energy
A measure of the ability to do work. Energy can be transferred. indifferent ways including heat, light, sound and electricity.
System
The area of interest
Surroundings
Everything else in the universe
Law of conservation of energy
The total energy of the system and surroundings cannot change during a process but heat can be transferred between a system and its surroundings.
Enthalpy
Chemical potential energy of a system
What happens to enthalpy when heat is added to the system?
The enthalpy of the system increases.
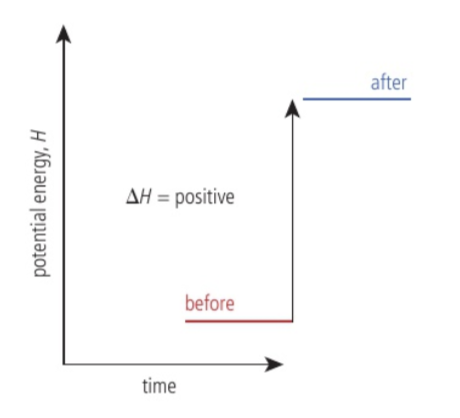
What happens to enthalpy when the system gives. outheat?
The enthalpy decreases.
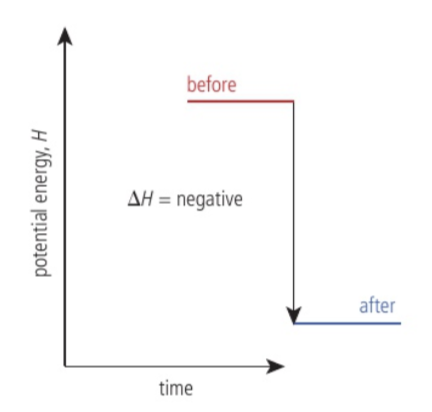
Exothermic reaction
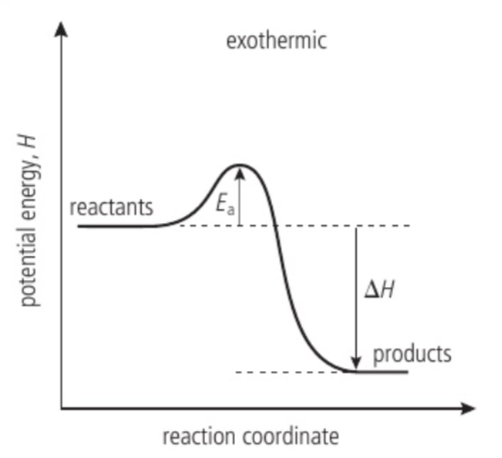
Give out heat to the surroundings and the enthalpy of the reaction is negative. For example, combustion. andall neutralization reactions.
Endothermic reaction
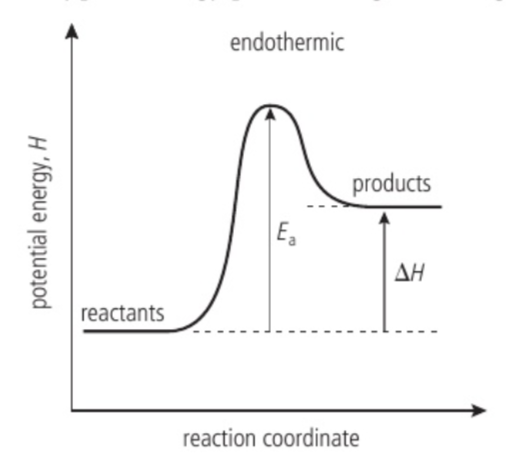
Heat transfer from the surroundings to the system. The products have more stored potential energy than the reactants and the enthalpy change is positive.
Enthalpy change in terms of temperature
Any enthalpy changes in the reaction are best observed by changes in the temperature of the water, which is acting as the solvent. In an exothermic reaction, the heat released is transferred to the solvent. The temperature increases as the water molecules increase their average kinetic energy and heat is transferred from the water to the wider surroundings as the system returns to its initial temperature.
Energy profile of an endothermic reaction
Energy profile of an exothermic reaction
Standard enthalpy change for a reaction
Refers to the heat transferred at constant pressure under standard conditions and states. It can be determined from the chnage in temperature of a pure substance.
Specific heat capacity
The heat needed to increase the temperature of a unit mass of a substance by 1 K. Depends on the number of particles present in a unit mass sample, which depends on the mass of the individual particles. Q =mCT
Calorimeter
Used to measure the enthalpy changes of combustion. The heat given out by the flame during the combustion reaction is used to heat a known mass of water in a metal calorimeter. Copper is often used as it is a good conductor of heat. The heat absorbed by the water can be calculated from its temperature change and its mass. The heat absorbed by the calorimeter can also be calculated from its heat capacity.
Enthalpy changes of a reaction in solution
can be calculated by carrying out the reaction in an insulated system such as a polystyrene cup
the heat released or absorbed can be measured from the temperature change in the water which is acting as a solvent.
Calorimeter is made from an insulator to maximize the amount of heat that is transferred between the reaction and the water in the system.
Source of error in experiments conducted in a polystyrene cup
Heat loss to surroundings.
Assumptions made for extrapolating the cooling section of the temperature time graph to determine the maximum temperature.
no heat loss from the system
all the heat goes from the reaction to the water
the solution is dilutre
water has a density of 1.00gcm-3
Bond enthalpy
Energy needed to break one mole of bonds inn gaseous molecules under standard conditions.
Bond enthalpy of Cl2
Cl2 —> 2Cl Bond enthalpy = +242kjmol-1
Making bonds
Exothermic process. The same amount of energy absorbed when a bond is broken as is given out when a bond is formed. Potential energy decreases.
Endothermic reaction in terms of bond strength
A reaction is endothermic when the bonds broken are stronger than the bonds formed.
Hess’s law
The enthalpy change for a reaction is independent of the pathway between the initial and final states.
Standard enthalpy change of combustion
The enthalpy change that occurs when one mole of the substance burns completely under standard conditions.
Standard enthalpy change of formation
The enthalpy change that occurs when one mole of the substance is formed from its elements in their standard states. This gives a measure of the stability of a substance relative to its elements and can be used to calculate the enthalpy changes of all reactions.
Born-Haber cycle
An application of Hess’s law, used to show energy changes in the formation of an ionic compounds.
First ionization energy
The energy needed to form the positive ion from a gaseous atom. It is an endothermic process as it involves pulling an electron away from the attractive electrostatic force due to the positively charged nucleus. Example:
Na—> Na+ + e- .
First electron affinity
Enthalpy change when one mole of gaseous atoms attracts one mole of electrons. As the electron is attracted to the positively charged nucleus of hte Cl atom, the process is exothermic. Example:
Cl + e- —> Cl-
Lattice enthalpies
The oppositely charged gaseous ions come together to form an ionic lattice. This is an exothermic process as there is a strong attraction between the oppositely charged ions.
Steps for the formation of a solid lattice
Atomization (turn solids into gases)
Bond enthalpy (split atoms if it is like O2)
Ionization (Form a positive ion)
Electron affinity (Form a negative ion)
Lattice enthalpy (from the products into the gaseous ions), normally flipped
Relationships of lattice enthalpy
proportional to the product of the charges on the positive and negative ions
inversely proportional to the sum of the ionic radii of the positive and negative ions
lattice enthalpy is greater for ions with a larger charge density as they have a small radius and are highly charged
Combustion reaction
A reaction in which an element or compound burns in oxygen. Involve electron transfers to oxygen.
What occurs during combustion based on the block?
The s block metals form ionic oxides which are basic and p block non-metals form covalent oxides which are acidic.
Combustion of organic compounds
Hydrocarbons and alcohols are used as fuels because their combustion reactions release energy at a reasonable rate to be useful. The complete combustion of organic compounds breaks the carbon chain and results in the production of carbon dioxide and water and the release of a lot of heat energy.
Spontaneity of the combustion of organic compounds
They do not spontaneously combust because the reaction has a sufficiently high activation energy so the fuels can be safely transported and stored.
Incomplete combustion
Happens if the supply of air is limited or when the compound has a high percentage of carbon content. Releases less heat than a complete combustion, thus the enthalpy of formation of carbon dioxide is more exothermic than that of carbon monoxide or soot.
Products of incomplete combustion
Carbon monoxide and carbon in the form of soot.
Catalytic converters
Used in cars to fully oxidize carbon monoxide to carbon dioxide and to convert unburnt and partialy burnt fuel hydrocarbons to carbon dioxide and water.
Atomization
Solid to gas
Reminder about electron affinity and ionization
Sometimes you have to do it twice, check the values in the data booklet for the first and second.
Fossil fuels
Non-renewable and non-sustainable energy sources.
Comparison of fossil fuels
octane is a main component of gasoline or petrol and it has a high energy density
Methane is preferred to hydrogen in pipelines for the same reason
When the mass of the fuel is important, hydrogen is a better choice with its high specific energy
Hydrogen is the only fuel which does not produce the greenhouse gas carbon dioxide.
Natural gas
Methane is the primary constituent of natural gas, which also contains nitrogen and sulfur compounds as impurities. Natural gas is the cleanest of the fossil fuels to burn due to its low percentage carbon content.
Coal advantages
cheap and plentiful
longest lifespan
can be converted into synthetic liquid fuels and gases
safer than nuclear power
Ash produced can be used in making roads
Coal disadvantages
contributes to global warming due to CO2
contributes to acid rain, SO2
Produces particulates
Difficult to transport
Waste can lead to visual and chemical pollution
Mining is dangerous
Petroleum advantages
easily transported in pipelines or by tankers
convenient fuel for use in cars as volatile and burns easily
high enthalpy density
sulfur impurities can be easily removed
Petroleum disadvantages
limited life span and uneven world distribution
contributes to acid rain and global warming
transport can lead to pollution
carbon monoxide is a local pollutant produced by incomplete combustion of gasoline in internal combustion engines
photochemical smog is produced as a secondary pollutant due to reactions of the primary pollutants released from internal combusion engines
Natural gas advantages
higher specific energy
clean: produces fewer pollutants per unit energy
easily transported in pipelines and pressurised containers
does not contribute to acid rain
Natural gas disadvantage
contributes to global warming
limited supplies
risk of explosion due to leaks
Incomplete combustion in alkanes
the higher the carbon content of the compound, the greater the tendency for incomplete combustion.
incomplete combustion increases with the length of the carbon chain
What the amount of carbon dioxide gas produced by a fuel is dependent on
The mass of carbon dioxide produced per unit mass increases with teh percentage carbon content
Greenhouse gases
Greenhouse gases allow shortwave radiation from the Sun to pass through the atmosphere but absorb the longer wave infrared radiation re-radiated from the Earth’s surface.
Carbon dioxide as a greenhouse gas
Its molecules increase their vibrational energy by absorbing infrared radiation. Three of the vibrational modes of the carbon dioxide molecule are IR active - the dipole changes as it vibrates. The molecules then re-radiate this energy back to the Earth’s surface, contributing to global warming.
Impact of greenhouse gas on climate
Increase in temperature of the earth causing:
changes in agriculture such as crop yields
changes in biodistribution due to desertification and loss of cold-water fish habitats
rising sea level because of thermal expansion and the melting of polar ice caps and glaciers
Biofuels
Fuels produced from the biological fixation of carbon over a short period of time through photosynthesis. Renewable and sustainable energy source.
Photosynthesis
Converts light energy into chemical energy. Energy from the Sun fuels the sequence of redox reactions that is photosynthesis.
Ethanol as a biofuel
Is made from biomass by fermenting plants high in starches and sugars. Is carried out at 37 degrees in the absence of oxygen by yeast, which provides an enzyme to catalyze the reaction.
Advantages of using ethanol
it is renewable
it produces lower emissions of carbon monoxide and nitrogen oxides
it decreases a country’s dependence on oil
Disadvantage of using ethanol
Ethanol absorbs water as it can form hydrogen bonds with the water molecules in the atmosphere. This leads to the ethanol separating from the hydrocarbon components in the fuel and can cause corrosion.
Methane as a biofuel
Made from the bacterial breakdown of plant material in the absence of oxygen.
Advantages of biofuels
cheap and readily available
if crops/trees are replanted, they can be renewable and sustainable source
less polluting than fossil fuels
Disadvantages of biofuels
uses land which could be used for other purposes such as growing food
high cost of harvesting and transportation in large volumes
takes nutrients from soil/uses large amounts of fertilizers
lower specific energy than fossil fuels
Fuel cell
Used to convert chemical energy from fuel directly to electrical energy.
The hydrogen-oxygen fuel cell with an alkaline electrolyte
electrons are made out of porous carbon with added transition metals such as nickel
potassium hydroxide provides the hydroxide ions that are transferred across the cell
Methanol fuel cell
the methanol is oxidized using catalysts in the methanol fuel cell
H+ ions formed are transported from the anode to the cathode where they react with oxygen to produce water
electrons are transported through an external circuit from the anode to the cathode
water is consumed at the anode and produced at the cathode
Difference between a fuel cell and a primary voltaic cell
Fuel cells do not run out, the fuel is supplied continuously to the cell as it is oxidized. In a primary cell, the chemicals are contained so it stops working when the redox reaction which generates the current is complete.
Entropy
A measure of the dispersal or distribution of matter in a system (disorder).The entropy of a gas is greater than than of a liquid, which in turn is greater than that of a solid.
Absolute values of entropy
Always positive.
Change in Gibbs energy
Relates the energy that can be obtained froma. chemical reaction to the change in enthalpy, change in entropy and absolute temperature.
The reason exothermic reactions are more common
There is an increase in entropy of the surroundings in exothermic reactions.
Calculating change in entropy
-change in enthalpysystem / T
Relationship between Gibbs energy and spontaneity
The change in Gibbs energy of a system must be negative for a spontaneous process.
Change in Gibbs Energy
Gives a measure of the quality of energy available. It is a measure of the energy which is free to do useful work rather than just leave a system as heat. Spontaneous reactions have negative Gibbs energy changes because they can do useful work.
Using change in G to predict how a system changes as the temperature is changed
HSystem < TSsystem
at low temperatures when TSsystem is approximately - this condition is met for all exothermic reactions as Hsystem < 0
Endothermic reactions which have a positive value of S can be spontaneous at higher temperatures when TS > H
The temperature at which an endothermic reaction becomes spontaneous can be determined from the equation
Tspontaneous = H/S
G and equilibrium
As a reaction approaches equilibrium, G becomes less negative and finally reaches 0.
Entropy and equilbirum
The total entropy reaches a maximum when we have equal amounts of reactant and product.
The equilibrium mixture when G is negative
at the start of the reaction, the total G of the reactants is greater than the products so the reaction proceeds int he forward direction and Q increases
As the reaction continues converting reactants to products, the G of the system decrease until it reaches equilibrium. Q equals the equilibrium constant.
Once equilibrium is reached, all possible changes would lead to an increase in G and a decrease in the total entropy of the universe and do not happen.
The position of equilibrium corresponds to a mixture with more products than reactants.
Relationship between K and G
G = negative K > 1 and equilibrium mixture is mainly products
G = positive K < 1 and equilibrium mixture is mainly reactants
G = 0 K=1 and equilibrium mixture is appreciable amounts of both reactants and products
Extent of the reaction if G > + 30kjmol-1
Spontaneous change impossible: no reaction. K is approximately 1
Extent of the reaction if 0kjmol-1 < G < +30kjmol-1
Partial reaction producing equilibrium mixture. K < 1
Extent of the reaction if G = 0kjmol-1
Partial reaction producing equilibrium mixture. K = 1
Extent of the reaction if 0kjmol-1 > G > +30kjmol-1
Partial reaction producing equilibrium mixture. K > 1
Extent of reaction if G < -30kjmol-1
Complete reaction. K >> 1
Ways to reduce the environmental impact of energy production from coal
remove sulfur from coal
add lime during combustion
not allow sulfur oxides to be released into the environment
reduce percentage of energy produced by the combustion of coal
Standard hydration enthalpy
The enthalpy of hydration is the energy change when 1 mol of an anhydrous compound turns to 1 mol of a hydrated compound.