Unit 10 - Lesson 3: Concentration of Solutions
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Concentration:
referring to the amount of substance within a defined area; typically associated with solutions
In the Sciences, there are multiple common units of concentration:
Molarity
Molality
Weight Percent
Mole Fraction
Molarity (M)
- [Moles of Solute] / [Liters of Solution] - We’ll be focusing on this one entirely…
number of moles of solute per liter of solution. (Represented by ‘M’)
How to Prepare a Molar Solution
Determine the Concentration (Molarity) of the solution that you want.
Decide the volume of your solution and use the corresponding Volumetric Flask. 1L = 1L Volumetric Flask, 500mL = 0.5L Volumetric Flask, etc.
Using a funnel, add some (NOT ALL) of your solvent to the Volumetric Flask. You want just enough so that you have enough solvent to dissolve the solute.
Gradually incorporate all of your solute so that it is now with the partially filled volumetric flask. Add more water until the volume of the solution is near the neck of the volumetric flask. (Use a wash bottle to ensure all solute is inside)
Remove the funnel and place a rubber stopper on the volumetric flask and swirl to create the initial mixture.
Remove the rubber stopper and then carefully add the remaining water until you reach the 1.0L mark (or whichever size volumetric flask you are using.)
7. Replace the rubber stopper on the flask and invert/revert the solution around ten times to ensure adequate mixing has taken place.
8. You now have your prepared solution!
Formula for Molar Solution (on check sheet)
M=mol/L
EXAMPLE:
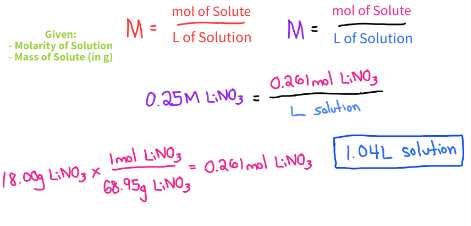
What volume of 0.250M LiNO3 is needed for a reaction that requires 18.0g of LiNO3?
EXAMPLE
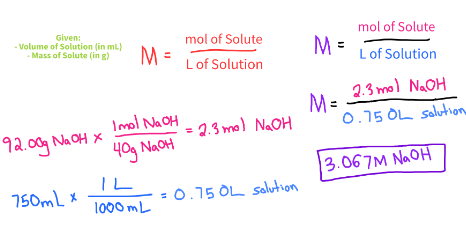
You have 750.0mL solution that contains 92.00g of Sodium Hydroxide. What is the Molarity of the solution?