3:Pharmacology of the Peripheral Noradrenergic neurotransmission
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
63 Terms
‘catecholamines’ examples
Noradrenaline (NA), adrenaline (Adr) and dopamine (DA)
Catecholamine structure
-All possess a catechol moiety (aromatic ring with two adjacent -OH groups) separated from an amine (-NHR, where R = H or alkyl group) group by a two carbon linkage
dopamine (DA)
-Has an alcoholic –OH at β position.
-Its a precursor of NA
-Important transmitter mainly in the CNS and dopaminergic nerves
What is the difference between adrenaline and noradrenaline?
NA which is effectively Adr without the methyl (-CH3) group at its amino nitrogen (N)
Noradrenaline (NA)
It is the main neurotransmitter in the sympathetic nervous system as well as a major neurotransmitter in the CNS
Adrenaline
Made in the adrenal gland and acts as a circulating hormone rather than as a neurotransmitter in the periphery and it is effectively the N-methylation of NA by PNMT (phenylethanolamine N-methyltransferase)
Isoprenaline
Synthetic (= N-isopropyl) derivative of NA, often used experimentally
Adrenaline synthesis pathway
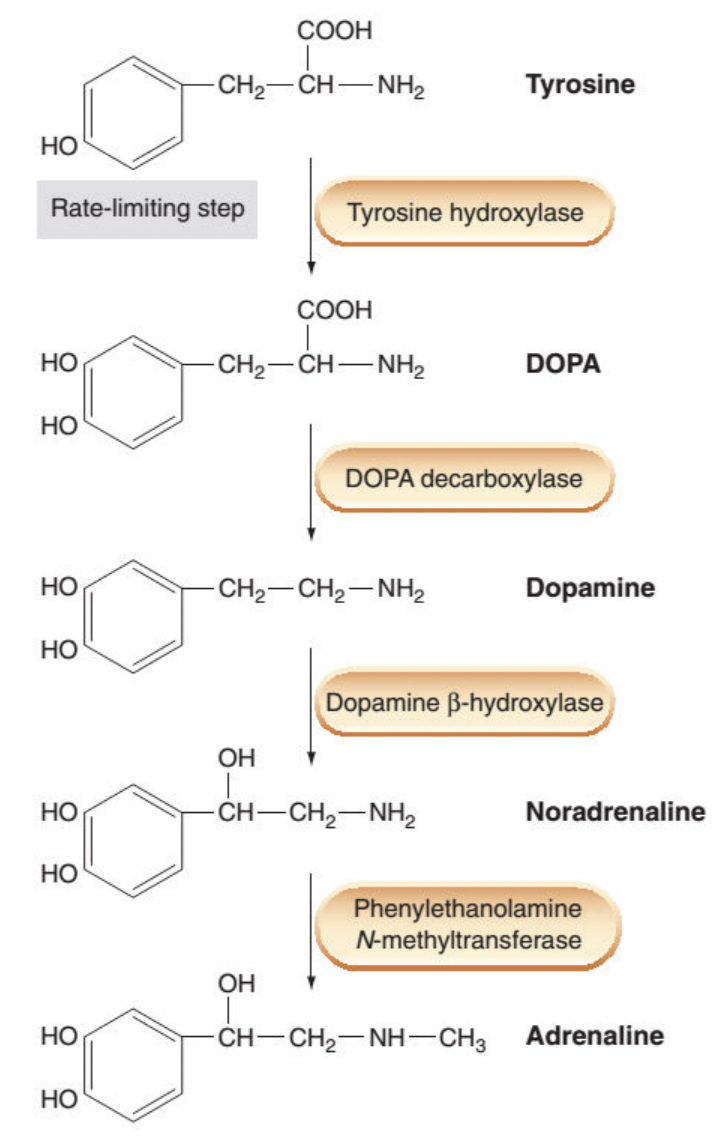
What is the start of catecholamine biosynthesis?
-L-tyrosine is present in body fluid and taken up from the circulation by catecholaminergic nerves.
-L-tyrosine is then converted into DOPA (dihydroxyphenylalanine) by tyrosine hydroxylase (TOH).
-This is the rate limiting step, TOH is exclusive to catecholaminergic nerves.
What happens after DOPA formed in catecholamine biosynthesis?
-Decarboxylation of DOPA to form dopamine (DA) and this is catalyzed by DOPA decarboxylase.
-The enzyme is a L-aromatic acid decarboxylase since it’s relatively non-selective and catalyzes decarboxylation of other L-aromatic acids.
What happens after dopamine is formed in catecholamine biosynthesis?
-After formation, DA is transported into vesicles via the vesicular monoamine transporter (VMAT)
-The process is driven by the transvesicular H+ gradient maintained by a V-type ATPase.
-The enzyme dopamine β-hydroxylase (DBH) lies within the inner surface of these vesicles (of noradrenergic nerves exclusively) and it catalyzes conversion of DA into NA through addition of –OH group at its beta-carbon.
What affects the expression of PNMT?
-The expression of PNMT is under the influence of glucocorticoids such as cortisol ('stress hormone') produced by the surrounding adrenal cortex.
-Reduction in cortisol level reduces PNMT expression, thus the Adr:NA ratio.
α-Methyltyrosine
A competitive inhibitor of TOH and is used experimentally to block NA production.
Because of being the rate limiting step, inhibiting this step substantially reduces the amount of transmitter produced.
How can we increase the production of the catecholamines?
Rate limiting step catalyzed by TOH needs to be bypassed through using the substrate for one of the later enzymes in the pathway.
This strategy is used in Parkinson's disease where dopaminergic neurons in the mid brain are selectively degenerated.
L-DOPA
-Use of exogenous DA is futile as it does not cross the BBB but its natural precursor, LDOPA readily does.
-Therefore L-DOPA is used to boost DA synthesis in the Parkinsonian brain.
-A substantial fraction of the administered L-DOPA undergoes decarboxylation in the periphery by peripheral decarboxylase to produce DA and NA which account for some of the unwanted effects associated with L-DOPA therapy.
-This is why often L-DOPA is used in combination with carbidopa which itself cannot cross the BBB but inhibits the peripheral decarboxylase to ensure that DA synthesis from L-DOPA occurs predominantly in the CNS where it is needed.
Disulfiram
-A drug used as an adjunct in the treatment of alcohol dependence, can be used experimentally to inhibit DBH.
-The basis of its action in dealing with alcohol addiction is however the inhibition of a different enzyme.
False transmitters
Production and storage of a variety of other amines in the synaptic vesicle occurs due to TOH, DDC and DBH being relatively non-selective in their substrate choice.
α-Methyldopa
Taken into noradrenergic nerve terminals and converted successively into α-methyldopamine (α-methyl DA), and αmethylnoradrenaline (α-methyl NA), respectively.
α-methyl NA is stored within the synaptic vesicles and released with (or in place of) NA as a false transmitter
False transmitter limitations
It is functional but compared to NA, it is less active at α1 adrenoceptors and more selective towards α2 adrenoceptors like another drug clonidine
α-methylnoradrenaline and clonidine effects
Cause a fall in blood pressure, partly by inhibition of NA release (through stimulating presynaptic α2 adrenoreceptors, see later) and partly by a central action
Vesicular storage of catecholamines & its pharmacological modulation
-Within the synaptic vesicles (typically clear-cored), NA is stored with ATP. NA (and DA) accumulates into these vesicle by the vesicular monoamine transporter (VMAT, a member of the SLC protein superfamily).
-The process is driven by the trans-vesicular H+ gradient which is set up by an active proton pump (a v-type ATPase).
What are the types of VMAT?
-Of the two existing VMAT isoforms, VMAT1 is mainly restricted to the periphery whilst VMAT2 is the major isoform in the CNS.
What happens when VMAT is inhibited?
Inhibition of VMAT blocks the uptake of monoamines into the synaptic vesicles that fail to be refilled with the catecholamines and this eventually depletes the nerves of these neurotransmitters.
Reserpine
A naturally occurring alkaloid (not used clinically) irreversibly inhibits both VMAT isoforms.
tetrabenazine
-FDA-approved drug
-inhibit reversibly and manifest more selectively towards VMAT2.
-These drugs (with suffix 'benazines') help in managing uncontrolled, repetitive and painful motor movements due to some diseases (Tardive dyskinesia, Huntington's chorea) or side effects of some drugs
Release of catecholamines & its
Catecholamines are released from presynaptic nerves through Ca2+-dependent exocytosis in response to arrival of the action potential and subsequent further depolarisation that results in opening of voltage-gated Ca2+ channels.
Pharmacological modulation of catecholamine release
Modulated by several ways including:
1) direct blockade of catecholaminergic neurons
2) triggering release in absence of any depolarisation (indirectly-acting and mixed-acting sympathomimetic drugs) and
3) through stimulating pre-synaptic receptors.
Direct blockade of adrenergic neurons.
Guanethidine (and there are few similar agents)
Guanethidine
Accumulates selectively into noradrenergic nerves via NET
Has complex, not clearly-understood but selective action on noradrenergic nerves.
At low doses, blocks impulse conduction in these nerves, like the local anaesthetics. It also accumulates into the synaptic vesicles via VMAT, causing gradual and long-lasting depletion of NA (like reserpine).
At a very high dose, it irreversibly damages the nerve and its action remains selective to noradrenergic neurons – this is exploited for inducing pharmacological sympathectomy in experimental studies.
What was the previous use of guanethidine?
Guanethidine was used previously to treat uncontrolled hypertension but it is now obsolete in the UK.
Indirectly-acting sympathomimetic amines
-Tyramine (a dietary amine)
-Dexamfetamine
Indirectly-acting sympathomimetic amines MOA
-Trigger catecholamine release from noradrenergic nerves without requiring depolarisation.
-These agents do not stimulate adrenoceptors (i.e. no agonist action) but are excellent substrates for monoamine transporters.
-They are avidly taken up into noradrenergic nerve endings by NET.
-In the presynaptic nerve endings, they are then loaded into vesicles via VMAT, displacing NA. The latter then leaks out of the vesicles, undergoes metabolism (by MAO) in the cytoplasm but can also escape (by reverse transport via NET) the nerve termini and be released into the synaptic/junctional space.
-Dexamfetamine is used in narcolepsy.
Tyramine-rich food
Tyramine-rich food intake in presence of MAO inhibition can lead to severe hypertensive crisis (see later). These types of agents are CNS stimulant and as such few (e.g. MDMA or 'ecstasy') are often abused for recreational purposes.
Mixed-acting sympathomimetic amines
-ephedrine
ephedrine MOA
Indirectly release NE like tyramine and dexamphetamine but they also can directly activate adrenoreceptors.
Ephedrine is used as a nasal decongestant as it results in NA-mediated vasoconstriction in some blood vessels to the nose.
It may have some direct action on bronchial β2-ARs and thus can help in relaxing the airways.
It is also used to treat urinary incontinence in female dogs after the surgical removal of their reproductive organs.
Presynaptic/prejunctional regulation of catecholamine release
At the noradrenergic nerves the most prominent effect is mediated by α2-adrenoceptors (coupled to Gi/o) which, when stimulated, decrease the amount of NA released.
Selective blockade and stimulation of these adrenoreceptors (ARs) leads to enhanced and reduced release of NA, respectively.
In few cases, there can be presynaptic/prejunctional β2-ARs (coupled to Gs) which upon stimulation, enhance NA release.
α2-AR inhibition and augmentation
Both inhibition and augmentation could be brought about by actions on adenylyl cyclase: α2-ARs stimulation decreases cAMP production while β2-ARs would increase it. Increased cAMP level within the presynaptic/prejunctional nerve termini, enhances Ca2+ channel activity in a PKA-dependent manner.
Stimulation of the α2-ARs, via the liberated subunit, also results in the opening of K+ channels (GIRKs), thus reducing the excitability of the nerve terminal via hyperpolarisation.
As both the predominant α2-ARs and the occasional β2-ARs interact with the NA released from the nerve ending on which they are situated - they serve as the autoreceptors.
There can be presynaptic/prejunctional heteroreceptors (e.g. muscarinic M2 and δ-opioid receptors) which upon activation by cognate agonists, decrease NA release.
Fate of the released catecholamines: removal from the synaptic/junctional space
Catecholamines are inactivated by transport processes removing them from the synaptic/junctional cleft. There are two processes involved:
-one into the presynaptic neuron (Uptake 1)
-the other into postjunctional effector cells (Uptake 2).
Uptake 1
-Primary importance as inhibiting this process enhances and prolongs the actions of released catecholamines
-Mediated by a transporter called NET (norepinephrine transport protein).
-Because VMAT has a much higher affinity for NE than does MAO (see later), most of the recaptured NE is resequestered into storage vesicles.
-In the periphery, NET is primarily responsible for the termination of NA action.
-There can be specific Uptake 1 transporter for specific transmitter and they are all targets of clinically-useful drugs: DAT for DA, SERT for serotonin and NET for NA.
NET (norepinephrine transport protein)
-NET is a member of the solute carrier (SLC) .
-It is Na+-dependent, and blocked by many drugs including cocaine and the tricyclic antidepressants such as imipramine.
-75% of the NA released by sympathetic neurons is recaptured by NET.
-Agents inhibiting NET (e.g. cocaine, tricyclic antedepressants such as imipramine) increase the longevity of the released NA at the synaptic/junctional cleft, making more available for stimulating its target adrenoceptors.
Uptake 2
-inhibiting Uptake 2 does not affect the response.
-The remaining of the released NA is captured by non-neuronal cells in the vicinity, limiting its local spread.
-Mediated by a transporter called ENT (extraneuronal amine transporter).
ENT (extraneuronal amine transporter)
ENT belongs to a largely and widely distributed family of Organic Cation Transporters (OCTs).
This is not Na+ -dependent and has a different pharmacological profile to NET.
Fate of the released catecholamines: metabolism
The catecholamines (either endogenously produced or exogenously administered including relevant drugs) are metabolised by two enzymes:
-monoamine oxidase (MAO)
-catechol O-methyltransferase (COMT).
-Enzymes can act on the catecholamines in any order to produce the final metabolites of catecholamines.
-A major such metabolite found in the urine is VMA (vanillylmandelic acid) that originates from the peripheral sources.
MAO
-Removes the amine group (-NHR) from the catecholamines and converts them into corresponding aldehydes (-CHO), which, in the periphery, are then rapidly metabolised into the corresponding carboxylic acids (-COOH).
-occurs in the outer membrane of mitochondria and is abundant in noradrenergic nerve terminals but also present in liver, intestinal epithelium and other tissues.
-Dietary amines such as tyramine is normally metabolized by MAOs in the gut and liver before reaching the systemic circulation.
-if people taking MAO inhibitors consume a large amount of tyramine-rich food (e.g. cheese, red wines etc.), then significantly higher amount of tyramine reaches the blood, is taken up into noradrenergic nerve termini via NET and eventually triggers release of NA.
-The latter can be sufficient to cause widespread vasoconstriction and a fatal hypertension; this is known to be the 'cheese effect'.
MAO in noradrenergic nerve termini
-Within the noradrenergic nerve termini, MAO degrades NA (and also DA) leaking from the synaptic vesicles
MAO in the liver
-Whilst MAO in the liver inactivates circulating monoamines such as tyramine.
MAO exists in two isoforms
-MAO-A
-MAO-B
-differ in their distribution, substrate specificity and pharmacology.
MAO-A
-degrades 5-HT, NA and DA
-Non-selective or selective MAO-A inhibitors are used in depression and anxiety disorders
MAO-B
-degrades DA more rapidly than the other monoamines.
-selective MAO-B inhibitors is used in the treatment of Parkinson's disease
COMT
-cytosolic enzyme that is expressed primarily in the liver and also found in the adrenal medullary chromaffin cells as a membrane-bound form but it is absent from the noradrenergic neurons.
-It methylates one of the aromatic hydroxyls (at position 3 of the aromatic ring) in the catecholamines(or their deaminated metabolites produced by MAO).
-The main end product of catecholamines following the sequential metabolism by MAO and COMT is VMA which is excreted via urine.
-In patients with tumours of chromaffin tissue of the adrenal medulla (Phaeochromocytoma - a rare cause of hypertension), the urinary excretion of VMA is markedly increased that helps in diagnosing the problem.
Entacapone
-Is an inhibitor of COMT which can be used in Parkinson's disease in combination with L-DOPA and Carbidopa.
-It reduces metabolism of L-DOPA in the periphery so that more L-DOPA could reach the CNS where it is needed to produce DA for the Parkinson’s patient and less side effects occur from activation of dopamine receptors present in various peripheral tissues.
Fate of the released catecholamines: action on adrenoceptors on target membranes
Catecholamine receptors in the periphery
Following release, NA and Adr bind to specific adrenergic receptors or adrenoceptors (ARs) which are GPCRs present in the membrane of the effector cells as well as the pre-synaptic nerve terminals. ARs are categorised into and family with several sub-classes emerged from each of the families. NA has higher affinity for the -ARs whilst isoprenaline (ISO) is a synthetic catecholamine with much higher affinity for -ARs. Agents directly acting on ARs can be agonists (= directly-acting sympathomimetics) and antagonists (= directly-acting sympatholytics). These agents can have variable degrees of selectivity towards AR subtypes. The overall effect of each of the endogenously-produced catecholamines and relevant drugs can be complex, often depending on the concentration of the agent as well as the density and relative proportions of and -ARs in specific tissues. Below we look at agents directly acting on ARs in context-specific way:
Eye
-alpha 1-ARs are present in the pupillary dilator muscles and their activation either by sympathetic nerve or adrenergic agonists causes mydriasis.
-alpha 1-ARs agonists (e.g. phenylephrine) can be used to induce mydriasis for diagnostic and therapeutic purposes (e.g. in anterior uveitis – an inflammatory condition of iris).
Blood vessels
Blood vessels to nasal mucosa express 1-ARs which upon stimulation produces vasoconstriction, thereby reducing congestion. For this reason, agonists ideally selective for 1-AR (e.g. phenylephrine, oxymetazoline etc.) or at least for -ARs (e.g. xylometazoline) are available as OTC drugs often as topical nasal decongestants for temporary relief of nasal congestion due to colds, hay fever or other upper respiratory allergies, or sinusitis. Note: drug names ending with ‘zoline’= -ARs agonists). Blood vessels supplied to skin, abdominal organs and kidneys also predominantly express 1-ARs which upon stimulation by physiological or synthetic agonists produces vasoconstriction. This increases peripheral resistance with resultant rise in blood pressure. The latter results in reflex bradycardia through baroreceptors that increases the vagal Rahman_Handout (relevant to Lecture 3) 6 tone. Antagonists selective against 1-ARs (often their names end with suffix ‘zosin’, e.g. prazosin) therefore are useful as anti-hypertensive agents. 2-ARs are present in blood vessels to skeletal muscles. Stimulation of these receptors physiologically (e.g. by adrenaline) or pharmacologically (e.g. by isoprenaline) causes vasodilation leading to decreased peripheral resistance and decreased diastolic pressure. Mean arterial blood pressure is only modestly increased and thus, baroreceptor reflex is not produced. 2-ARs in the periphery are often located presynaptically located and being Gi coupled, their activation reduces catecholamine and other neurotransmitter release in the periphery. Selective agonist for 2-ARs such as clonidine (you will use it in one of your MODA practicals) are useful as anti-hypertensive drugs. Clonidine reduces blood pressure, partly by inhibiting NA release from peripheral nerve endings but also by acting on central neurons that reduces sympathetic discharge towards the periphery. Another anti-hypertensive drug, α-methyldopa (or simply, methyldopa) which is taken up in the presynaptic nerve and gets converted into α-methylnoradrenaline (α-methylNA). The latter is released as a false neurotransmitter but like clonidine, it selectively activates pre-synaptic 2-ARs.
Heart
Briefly, sympathetic stimulation or adrenergic agonists increase heart rate and force of myocardial contraction and these are predominantly mediated through β1-ARs. These receptors are competitively antagonised by the β-blockers. Nonselective β-blockers (the first generation) act at both β1 and β2 -ARs (and presumably at β3-AR as well, although we know relatively little about this isoform), whereas cardioselective βAR antagonists (the 2nd generation) primarily block β1 -ARs. There are also 3rd generation of β-blockers that selectively target β1 -AR and an additional target(s) providing added clinical benefits – so they are essentially ‘polypharmacological by design’. β-blockers represent a widely used therapeutic class. They are primarily indicated in hypertension and angina but can be useful in cardiac arrhythmias, myocardial infarction, heart failure, hyperthyroidism, and glaucoma. Note: The names of all β-blockers end in ‘lol’ and many have “-olol” suffix
Lungs
The airway/bronchial smooth muscle cells are enriched in β2-ARs and selective agonists of these AR subtype relaxes the smooth muscle (= bronchodilatation). The effect is mediated through PKA activation and subsequent phosphorylation of MLCK which reduces its affinity for binding to Ca2+-Calmodulin. The ultimate outcome is relaxation of bronchial smooth muscle relaxation. Selective agonists of β2-AR (with various durations of actions) have important clinical use as bronchodilators in asthma and COPD
Urinary bladder
Bladder detrusor muscle is enriched with β3-ARs and their selective stimulation leads to detrusor muscle relaxation and increased bladder capacity. This action prevents voiding and provides relief for those with an overactive bladder and urinary incontinence. Therefore, β3-AR selective agonists (e.g. Mirabegron) are clinically useful against incontinence due to overactive bladder.
Prostrate gland:
Benign Prostatic Hyperplasia (BPH) is a common problem in a significant percentage of older men. The symptoms of BPH include a resistance to urine outflow. This results from mechanical pressure on the urethra due to an increase in smooth muscle mass and an α1-AR–mediated increase in smooth muscle tone in the prostate and neck of the bladder. Antagonism of α1-ARs by selective agonists (e.g. often drugs with suffix ‘zosin’ such as prazosin; you will use it in one of your practical classes) permits relaxation of the smooth muscle and decreases the resistance to the outflow of urine. However, these α1-AR selective antagonists also pose some risk for hypotension, especially due to their action on α1B-AR to regulate the vascular tone. In recent time, there has been more subtype selective agent namely α1A-AR selective antagonists (e.g. tamsulosin) which only work on bladder and come with no serious effect on blood pressure.