HL Chemistry: Unit 2
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Light
Believed to exist in tiny "packets" or photons, known to exhibit properties of both particles and waves, a form of electromagnetic radiation
Median
Matter needed for waves like sound to propagate
Why are wavelengths shorter than visible light dangerous?
More energy
Greater potential to ionize (break bonds in atoms, lifting it from a bond)
Wave propagation
The way waves travels
Wavelength (λ/lambda)
Distance over which the waves shape repeats, from crest to crest or trough to trough
Has periodicity
frequency
Number of waves that occur per second
Equations to calculate wave properties
c = λf
- Inverse relationship between wavelength and frequency
ΔE = hf
- ΔE: Change in energy
- h: Planck's constant (6.63 * 10-34 JS)
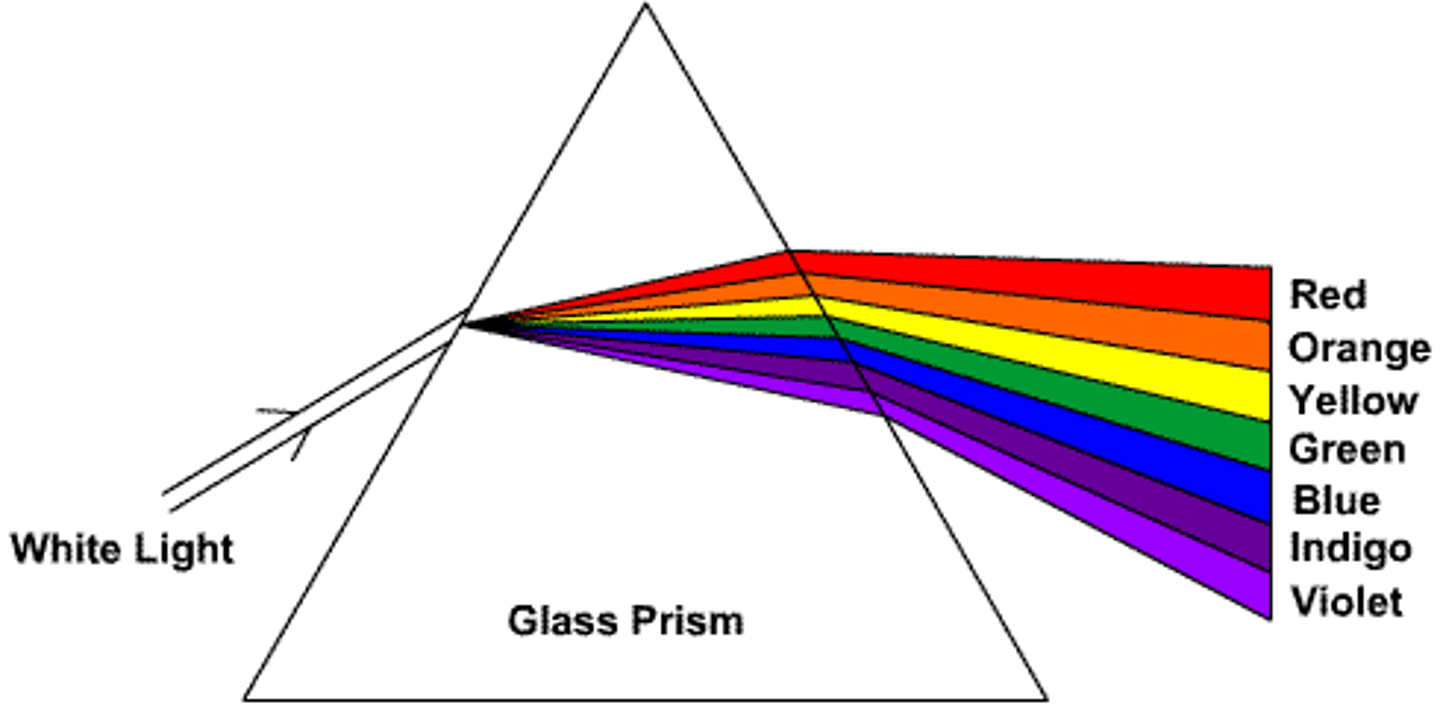
Diffraction grating
White light consists of all wavelengths of light, able to diffract light using a prism and able to see the wavelengths contained within
The diffracted rays from top to bottom go from longest to shortest wavelength
Continuous spectrum
Contains all wavelengths in a given region
Ex. White light, sunlight
Line spectrum
Lines of light or no light at specific wavelengths
Each line is ONE WAVELENGTH, ONE COLOUR, ONE FREQUENCY, ONE AMOUNT OF ENERGY
The emission and absorption spectrum of any given element have lines in identical locations
Emission spectrum
A gas is given energy until it glows, the emitted light from the hot gas is then passed through a prism, only specific wavelengths of light are emitted by the atoms in the gas
Absorption spectrum
A source of white light is passed through cold gas source then through a prism, atoms in the cold gas blocks/absorbs specific wavelengths of light
Ground State
State electrons in stable atoms and ions are in
Exciting Electrons
When energy is added, the electron is excited/promoted (heat, electricity, visible light) to a higher energy level, but the electron (the atom) does not want the extra energy. Promoted electrons will "drop" back down to lower energy levels until they reach the ground state, and the excess energy is "emitted" in the form of light.
- The fall/excitation of a specific distance will always emit/absorb the same specific amount of energy, we can only see some of this emission
- Greater the distance an electron jumps down, the greater the light energy emitted
Quantized/Discrete
Electrons are only found in specific/fixed energy levels in the atom
Specific/Limited number of amount of energy that the electron can lose or gain
Hydrogen spectrum
Ultraviolet region: n = 1
Visible region: n = 2
Infrared region: n = 3
Convergence
Lines get closer together as energy increases, frequency increases and wavelength decreases
Decreasing distance between lines of light on the spectra
Energy levels get closer together (converge) as the energy level number increases
Ionization energy
As n → ∞ reaches the limit to which convergence occurs, indicates the energy required to completely remove an electron from the atom
Graph and extrapolate to when change in energy lines converge to when difference is 0
Heisenberg's uncertainty principle
Within a given energy level, the exact position of an electron cannot be determined, electrons exist in a region of probability
Schrodinger's equation
Predicted that electrons are waves (differential calculus)
Electrons are also particles because they have mass and charge
Allows us to find the probability of finding an electron with a particular energy in a particular location
Answer to equation → electrons can be described by 4 distinct quantum numbers
Orbital
Areas of probability
Sub-energy level
Distance to nucleus
Principle quantum number
energy level (n)
Electron's average distance from the nucleus
Equal to number of different orbital types that exist at each energy level
Angular-momentum quantum number
shape/secondary distance of orbital (ℓ)
Maximum: ℓ = n - 1
The higher the ℓ value, the further it is from the nucleus (radius increases)
Each value of ℓ corresponds to the specific shape of the given orbital
"s" orbital
ℓ = 0
Spherical shaped
Angular-momentum
Can hold up to 2 electrons
As the value of n increases, the radius of the s-orbital increases
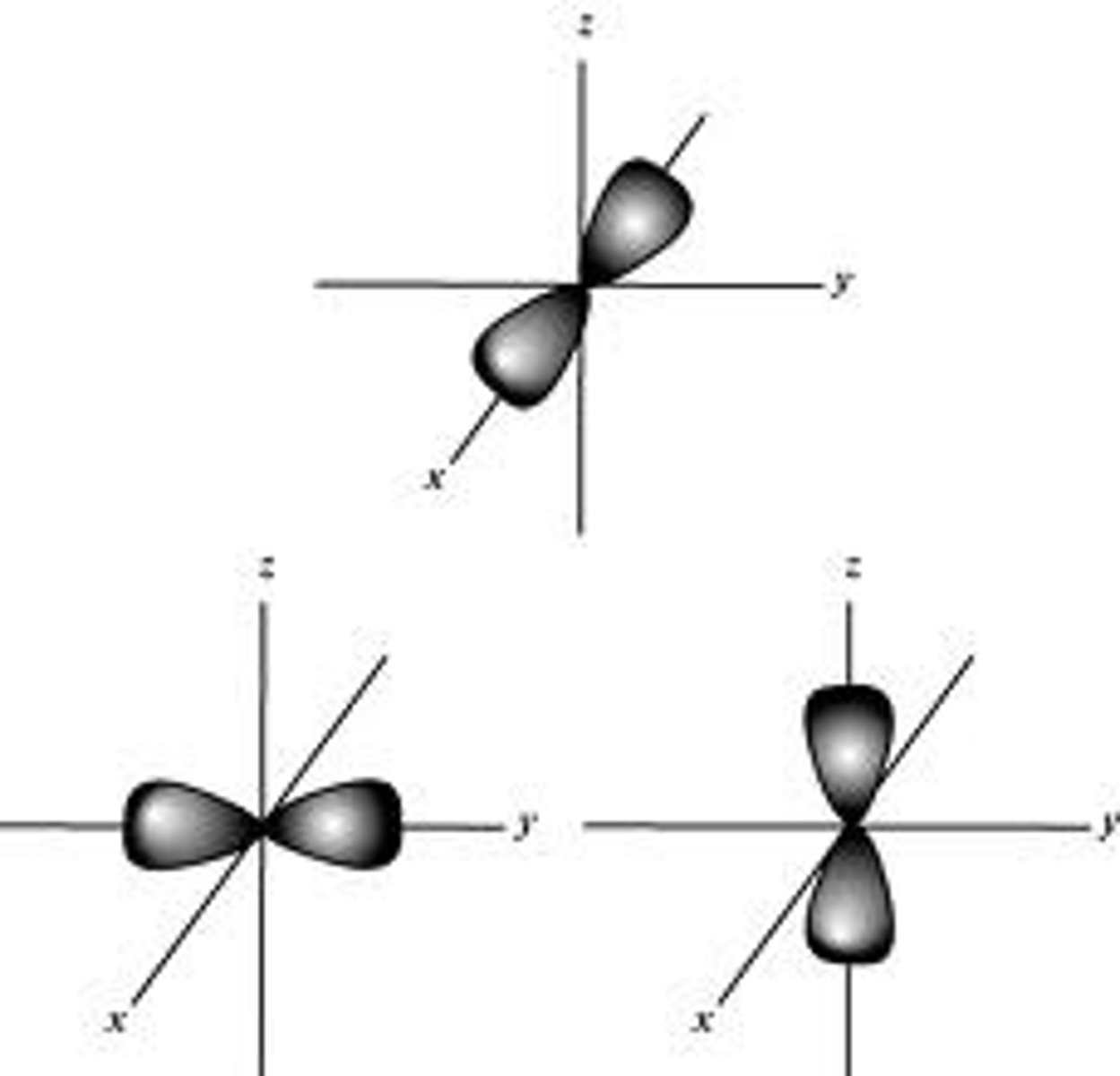
"p" orbital
ℓ = 1
Barbell shape
3 different orbitals/orientations (Px, Py, Pz) corresponding to 3-dimensions
Each orientation (orbital) can hold up to 2 electrons
Maximum of 6 electrons in the p (ℓ=1) sub-shell
All equally distanced from the nucleus
"d" orbital
ℓ = 2
Various shapes
5 different orbitals/orientations
Each orientation can hold up to 2 electrons
Maximum of 10 electrons in the d sub-shell
"f" orbital
ℓ = 3
Various shapes
7 different orbitals/orientations
Each orientation can hold up to 2 electrons
Maximum of 14 electrons in the sub-shell
Magnetic quantum number
orientation of orbital (mℓ)
Due to the angular momentum (rotation around the nucleus) electrons produce a magnetic field
The actual value of mℓ is determined by the chosen value of ℓ
mℓ = (-ℓ) ... 0 ... (+ℓ)
- ℓ = 1 → mℓ = -1, mℓ = ∅, mℓ = +1 (Px, Py, Pz)
Degenerate orbital
Orbitals within a sub energy level
Spin quantum number
(ms)
Refers to the characteristic of the electrons not the characteristics of the orbit
Electrons behave as though they are spinning
ms = +½ (clockwise spin)
ms = -½ (counter clockwise spin)
The first electron in an orbital always started with a positive ms value
Aufbau principle
Each additional electron added will occupy the next lowest available orbital
Overlap in some sub-shell due to convergence

Pauli exclusion principle
No electrons in an atom can have the same 4 quantum numbers
Hund's rule
Electrons in p, d, f orbitals can't be paired until each of the orbitals at the same sub-energy level contain at least 1 electron
Abbreviated notation of electron configuration
Start with the closest noble gas
Finish the configuration from there
Ex. Carbon 1s2 2s2 2p2 → [He] 2s2 2p2
Isoelectronic
Different elements with the same electronic configuration
To gain stability, atoms become isoelectronic with noble gases
Ex. F- (ion) 1s2 2s2 2p6 → Ne 1s2 2s2 2p6
Ions
Full, empty, or ½ filled sub-energy levels are also stable electron configurations
Ex. Zn: [Ar] 4s2 3d10 → it takes too much energy to lose 12 valence electrons
- Most d-block elements loses 2 of its "s" valence electrons to form form a 2+ ion, emptying its highest energy level
Ions Exceptions
Both copper and chromium "promote" one 4s electron a short distance to the 3d to gain half-filled and full sub-shell stability
3d and 4s shells are so close to each other, it requires little energy to be promoted, and 3d is almost filled, so it takes an electron from the 4s (it gets promoted), to give 1 half orbital and 1 full orbital
Copper: [Au] 4s2 3d9 → 4s1 3d10
Patterns
It takes more energy to remove an electron from a lower sub-energy level than a higher one (ionization energy)
- Lower energy levels are closer to the nucleus
- Electrons have less energy in lower levels
Removing an electron to reveal a stable state (full sub-energy level or ½ filled sub-energy level) is easier than removing an electron from a stable state
A more positive ion has a stronger attraction to (negatively charged) electron than a less positive ion
Ex. Despite the fact that B has more protons (nuclear charge) than Be, its first removed electron is further from the nucleus (and more shielding) and reveals a full sub-energy level → it is easier to remove B's 1st electron
As you go up in energy, difference between energy decreases → convergence
Transition Metals
Has an atom or at least one stable ion with incompletely filled d-orbitals (new)
All transition metals are in the d-block but not all d-block elements are transition metals
Ex. Atoms → Zn: [Ar] 4s2 3d10, Cu: [Ar} 4s1 3d10 (full d-sub-energy level → not a transition metal), Sc: [Ar] 4s2 3d1 (incomplete d-sub-energy level)
Ex. Ions → Zn2+: [Ar] 3d10 (full d-sub-energy level → not a transition metal), Sc3+: [Ne] 3s2 3p6 (empty d-sub-energy level)
Most transition metals can form a stable 2+ ion
Transition metal patterns going across period 4 d-block
Sc → Mn
- Max charge possible increases
- Number of possible ions increases
- Because there are more d electrons to lose
Fe → Zn
- Larger nuclear charge, more difficult to lose electrons
- Stability of paired d electrons
Oxidation state
Redox term: A reversible chemical reaction where one reaction is an oxidation and the reverse is a reduction
Often the same as charge
Charge (sign, #) vs. Oxidation state (#, sign)
Magnetic Properties
Unpaired d-electrons allow transition metals to have magnetic properties