Chemistry - 6 Electrolysis - 6.3 The Extraction of Aluminium & 6.4 Electrolysis of Aqueous Solutions
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Uses of aluminium (and its alloys) [7]:
- pans
- overhead power cables
- aeroplanes
- cooking foil
- drink cans
- window and patio door frames
- bicycle frames and car bodies
What compound is electrolysed to procure aluminium?
aluminium oxide, Al₂O₃
Where is aluminium oxide found?
bauxite ore
Aluminium oxide melting point
2050°C
How do we reduce the melting point of aluminium oxide?
mix it with cryolite
Cryolite-aluminium oxide mixture melting point
850°C
Aluminium oxide electrolysis reaction
2Al₂O₃ → 4Al + 3O₂
Aluminium forms at the ... electrode
negative
Oxygen is produced at the ... electrode
positive
Aluminium electrolysis cell [4]
- lined with carbon negative electrode
- molten aluminium is tapped or siphoned off
- carbon dioxide and oxygen gas emitted from anodes
- steel case
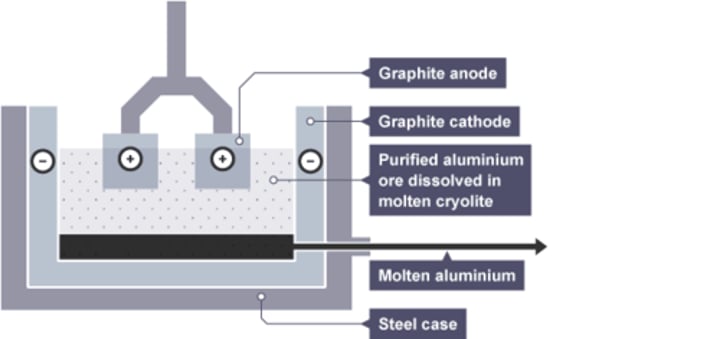
At the cathode (aluminium extraction):
Al³⁺ + 3e⁻ → Al
At the anode (aluminium extraction):
2O²⁻ → O₂ + 4e⁻
Reaction of oxygen with hot carbon anodes:
C + O₂ → CO₂
... have to be replaced regularly
Carbon anodes
Brine
water saturated with salt (sodium chloride)
Products of electrolysis of brine [3]
- chlorine gas
- hydrogen
- sodium hydroxide
At the anode (brine electrolysis):
2Cl⁻ → Cl₂ + 2e⁻
At the cathode (brine electrolysis):
2H⁺ + 2e⁻ → H₂
What is left after brine electrolysis?
sodium hydroxide, NaOH