2. Inorganic Chemistry
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
109 Terms
what are the formulas for the energy associated with 1 mole of photons
E = Lhf
E = Lhf/λ
how can electromagnetic radiation be described and characterised as
in terms of waves and in terms of wavelength and/or frequency
what is the formula for the relationship between wavelength and frequency
c = fλ
what are ways electromagnetic radiation can be described as
wave
particle
what is electromagnetic radiation said to have
dual nature
what happens when electromagnetic radiation is absorbed or emitted by matter
it behaves like a stream of particles with the particles being photons
what level of energy does a photon carry
energy proportional to the frequency of radiation
what happens when a photon is absorbed or emitted
energy is gained or lost by electrons in the substance
whats the difference between photons of higher frequency compared to those with lower frequency
can transfer a greater amount of energy
what are the formulas used for a single photon
E = hf
E = hc / λ
what might happen when energy is transferred to atoms
electrons within the atoms may be promoted to higher energy levels
when does an atom emit a photon of light
when an excited electron moves from a higher energy level to a lower energy level
what does light energy emitted by an atom produce
a sprectrum made up of a series of lines at discrete energy levels
what does each element create in terms of spectra
produces characteristic absorption and emission spectra so they can be used to identify and quantify the element
how is radiation absorbed in absorption spectroscopy
as electrons are promoted to higher energy levels
how is an absorption spectrum produced
by measuring how the intensity of absorbed light varies with wavelength
how are electrons excited in emission spectroscopy
high temperatures
how is an emission spectrum produced
an electron is promoted to a higher energy level
when it falls back to a lower state energy is emitted
how is the concentration of an element found in atomic spectroscopy
the intensity of light emitted or absorbed
how can the discrete lines observed in atomic spectra be explained
if electrons display the properties of both particles and waves
how do electrons behave
as standing waves in an atom, theyre waves that vibrate in time but dont move in space
what are orbitals
the different sizes and shapes of waves around the nucleus
how many electrons can an orbital hold
2
what different shapes of orbitals are there
s
p
d
f
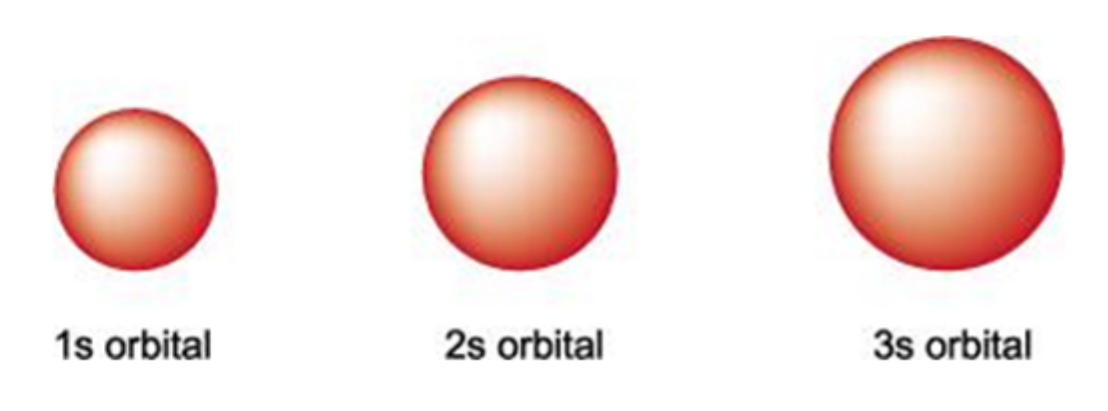
what is the shape of s orbitals
all spherically symmetrical
what happens to the electron density of s orbitals when the value of n increases
concentrated further out from the nucleus
how many electrons can be occupied in an s orbital
2
what value of n do p orbitals begin with
2
how many p orbitals do each principal energy shell contain
3
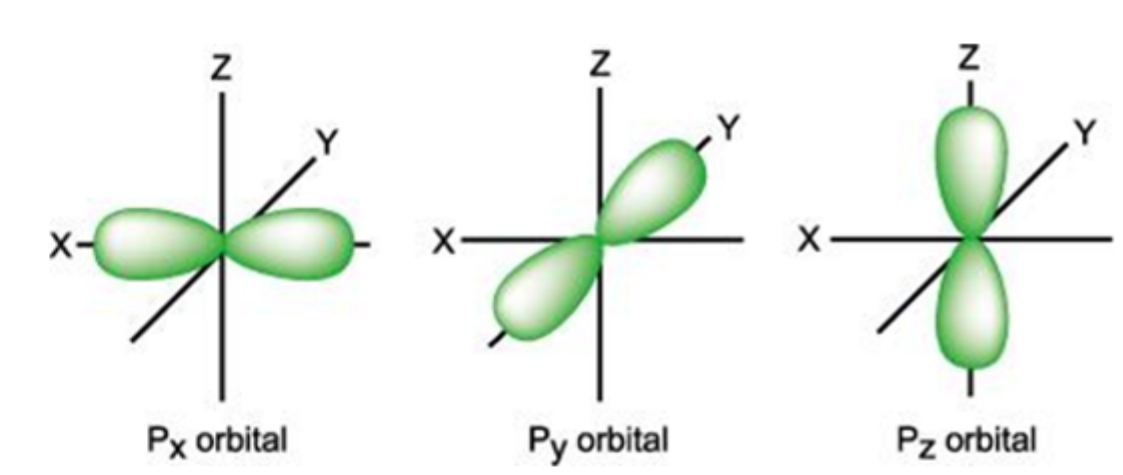
how can electrons in p orbitals be described
more difficult to describe as the probability of finding them isnt spherically symmetrical around the nucleus
for p orbitals where is the probability of finding an electron
concentrated in 1 direction
what does degenerate mean
of same/equal energy
what are the 3 degenerate p orbitals called
px, py, pz
at what angle do p orbitals lie
right angles of each other
how many electrons can a p orbital have
2 in each so maximum of 6
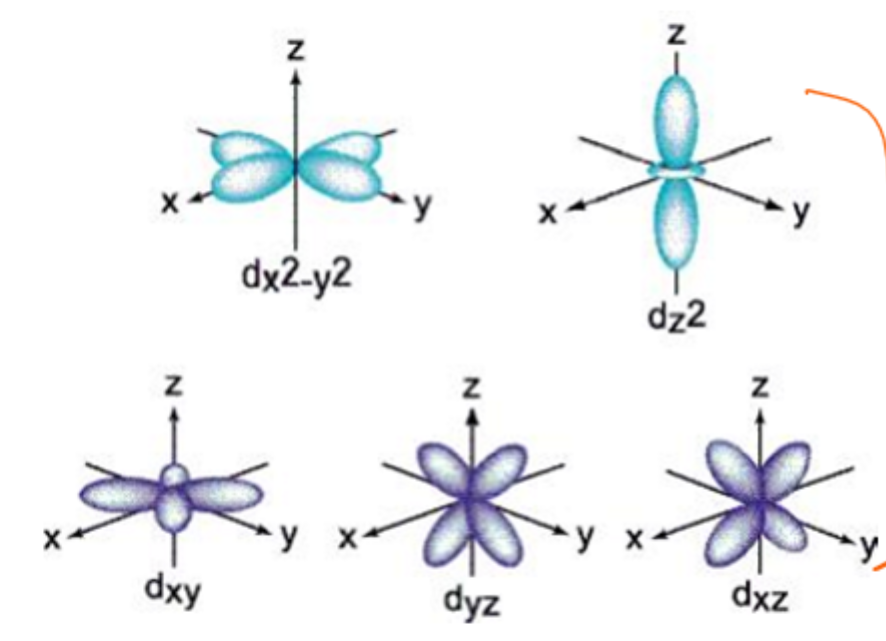
what value of n do d orbitals begin with
3
how many d orbitals are in each principal energy level
5
how many electrons can be held in a d orbital
2 electrons in each so maximum of 10
what are the shapes of each s orbital
what are the shapes of each p orbital
what are the shapes of each d orbital
what is quanta
electrons within atoms have fixed amounts of energy
what is the principal quantum number, n
the main energy level for an electron
how many electrons can be in each shell as n increases
2 when n = 1
8 when n = 2
18 when n = 3
32 when n = 4
what does the angular momentum quantum number, I do
determines the shape of the orbital in which the electrons are contained
what happens as the value of l increases
s subshell if l = 0
p subshell if l = 1
d subshell if l = 2
f subshell if l = 3
what happens to l when n increases
n = 1 so l = 0, the first shell has 1s subshell
n = 2 so l = 1, the second two subshells 2s, 2p
n = 3 so l = 2, the third shell has three subshells 3s, 3p, 3d
n = 4 so l = 3, the fourth shell has four subshells 4s, 4p,4d, 4f
what is the magnetic quantum number ml
determines the orientation of the orbital
what happens to the ml as l increases
l = 0 s subshells do not split, ml = 0 (one s orbital)
l = 1 p subshells split into three p orbitals - px, py, pz, ml = -1, 0, +1
l = 2 d subshells split into five d orbitals - dxy, dxz, dyz, dx2-y2, dz2, ml = -2, -1, 0, +1, +2
l = 3 f subshell split into seven f orbitals - ml = -3, -2, -1, 0, +1, +2. +3
what is the spin magnetic quantum number ms
determines the direction of spin
what values can ms have
+½ or -1/2
what does the aufbau principle principle state
electrons fill orbitals in order of increasing energy
what is hunds rule
when degenerate orbitals are available, electrons fill each singly, keeping their spins parallel before spin pairing starts
what is the pauli exclusion principle
no two electrons in one atom can have the same set of four quantum numbers, therefore, no orbital can hold more than two electrons and these two electrons must have opposite spins
what are all orbitals classed as in an isolated atom
degenerate
what are the 2 unusual elements due to their electronic configurations
chromium
copper
why are chromium and copper unusual
3d and 4s subshells are close together in energy and theres a special stability associated with all 5 d orbitals being half or completely filled
when do you know if an electronic configuration is in an excited state
if an atoms electronic configuration doesnt correspond to the one that would be given
what should you look out for when filling in electronic configurations
4s is filled in and empties before 3d
what is the periodic table divided into
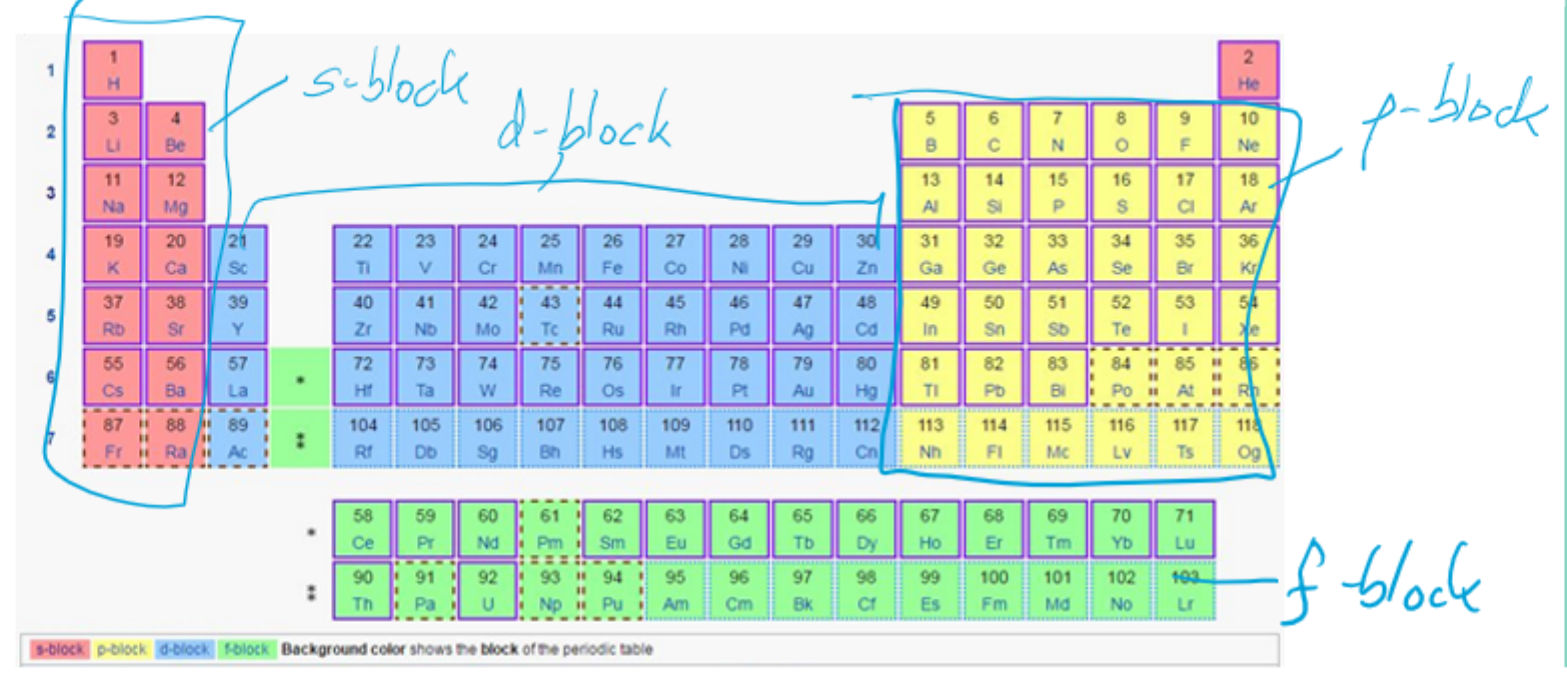
how can the variation in ionisation energies in the first 36 elements be explained
in terms of the relative stability of different subshell’s electronic configurations
what is the relationship between stability of an electronic configuration and ionisation energy
the more stable the electronic configuration, the higher the ionisation energy
what can VSEPR be used to predict
the shapes of molecules and polyatomic ions
what are the steps to finding the number of electron pairs surrounding a central atom
taking the total number of outer electrons on the central atom and adding one for each atom attached
adding an electron for every negative charge
removing an electron for every positive charge
dividing the total number of electrons by two to give the number of electron pairs
how are electron pairs arranged
in a way to minimise repulsion and maximise separation
what is the arrangement of electron pairs when there are 2 electron pairs
linear (180°)
what is the arrangement of electron pairs when there are 3 electron pairs
trigonal planar (120°)
what is the arrangement of electron pairs when there are 4 electron pairs
tetrahedral (109.5°)
what is the arrangement of electron pairs when there are 5 electron pairs
trigonal bipyramidal (120° and 90°)
what is the arrangement of electron pairs when there are 6 electron pairs
octahedral (90°)
how are the shapes of molecules or polyatomic ions determined
by the shapes adopted by the atoms present based on the arrangement of electron pairs
whats the order of electron pair repulsion in terms of decreasing strength
non-bonding pair/non-bonding pair > non-bonding pair/bonding pair > bonding pair/bonding pair
what are transition metals
metals with an incomplete d subshell in at least 1 of their ions
how can the oxidation number be found
uncombined elements have an oxidation number of 0
ions containing single atoms have an oxidation number that is the same as the charge on the ion
in most of its compounds, oxygen has an oxidation number of −2
in most of its compounds, hydrogen has an oxidation number of +1
the sum of all the oxidation numbers of all the atoms in a neutral compound must add up to zero
the sum of all the oxidation numbers of all the atoms in a polyatomic ion must be equal to the charge on the ion
what can oxidation be defined as in terms of oxidation number
an increase in oxidation number
what can reduction be defined as in terms of oxidation number
a decrease in oxidation number
what type of agent are compounds with high oxidation states
oxidising agents
what type of agent are compounds with low oxidation states
reducing agents
what do transition metal complexes consist of
a central metal atom/ion surrounded by ligands
what can ligands be
negative ions
molecules with non-bonding electron pairs
what happens to the non-bonding electron pairs in ligands
theyre donated to the central metal atom/ion forming dative covalent bonds
what are dative covalent bonds
a covalent bond where both electrons are donated by the same atom
what can ligands be classified as
monodentate
bidentate up to hexadentate
what does the coordination number mean
the total number of bonds from the ligands to the central transition metal
what is H2O called when naming complexes
aqua
what is NH3 called when naming complexes
ammine
why are ammonia and water classed as mondentate ligands
forms only one bond to the metal
what is CO called when naming complexes
carbonyl
what happens to the names of negative ions
“ide” should be changed to “ido”
“ite” or “ate” to “ito” or “ato”
how should ligands be listed
alphabetically by the name
what happens to the name of metals when the overall complex is negative
“ate” is added
what are the latin names used for metals in negative complexes
iron → ferrate
copper → cuprate
what happens to d orbitals in a complex of a transition metal
they no longer degenerate
how does the splitting of d orbitals into higher and lower energies occur
when electrons present in approaching ligands cause the electrons in the orbitals lying along the axes to be repelled
what are strong field ligands
ligands that cause a large difference in energy between subsets of d orbitals
what are weak field ligands
ligands that cause a small energy difference between subsets of d orbitals
what is the spectrochemical series
when ligands are placed in an order of their ability to split d orbitals
how can the colours of many transition metal complexes be explained
in terms of d-d transitions
how is light absorbed in transition metals
when electrons in lower energy d orbitals are promoted to a d orbital of higher energy
what happens when the light of 1 colour is absorbed
the complementary colour will be observed