Electrons In Atoms
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Scientific notation
a way to express a very large or small number
To convert a decimal into scientific notation, move the decimal until you have a number between 1 and 9.999
Place a x10 and add the exponent equal to the number of times you have moved the decimal
If the exponent is positive, the decimal has moved left
If the exponent is negative, the decimal has moved right
To put into the calculator, use the EE button to represent the x10
Standard notation
To convert scientific notation to standard, move the decimal as many times as the exponent
If the exponent is positive, the decimal moves right
If the exponent is negative, the decimal moves left
Light
Light is a kind of electromagnetic radiation
includes many types: gamma rays, x-rays, radio waves, etc
Speed of light = 2.998 x10^8m/s → abbreviated as “c”
All electromagnetic radiation travels at the same rate as all light when in a vacuum (no friction)
All light has different wavelengths
Equation for wavelengths
Equation: c = λⱱ
c = speed of light, constant, 2.998 x108m/s
λ (lambda) = wavelength, in meters
ⱱ (nu) = frequency in units of hertz (hz) or sec-1
Relationship between wavelength and frequency
Wavelengths and frequency are inversely related
As one goes up the other goes down
Small wavelengths = high frequency + more energy
Big wavelengths = low frequency + less energy
Different frequencies of light are different colors
There is a wide range of frequencies, and the whole range is called a spectrum
Energy
E=hⱱ
E = energy in joules (j) of a quantum of radiation
ⱱ is the frequency of radiation emitted in units of hertz (hz) or sec-1
h is a fundamental physical constant known as planck’s constant
h=6.626 x10-34 j*s
Atomic orbitals/sublevels
Within each energy level, the complex math of Schrodinger's equation describes several shapes
These are called atomic orbitals → regions where there is a high probability of finding an electron
Sublevels → like theater seats, arranged in sections
Letters: s (2), p (6), d (10), f (14)
Aufbau principle
electrons enter the lowest energy level first
This causes difficulties because of the overlab of orbitals of different energies
Pauli exclusion principle
at most two electrons per orbital - different spins
Hund’s rule
when electrons occupy orbitals of equal energy, they dont pair up intil they have to
Energy levels
First energy level
Has only 1 s orbital
Only 2 electrons
1s^2
Second energy level
Has s and p orbitals
2 in s and 6 in p = 8 total electrons
2s^2, 2p^6
Third energy level
Has s, p, and d orbitals
2 in s, 6 in p, and 10 in d = 18 total electrons
3s^2, 3p^6, 3d^10
Fourth energy level
Has s, p, d, and f orbitals
2 in s, 6 in p, 10 in d, and 14 in f = 32 total electrons
4s^2, 4p^6, 4d^10, 4f^14
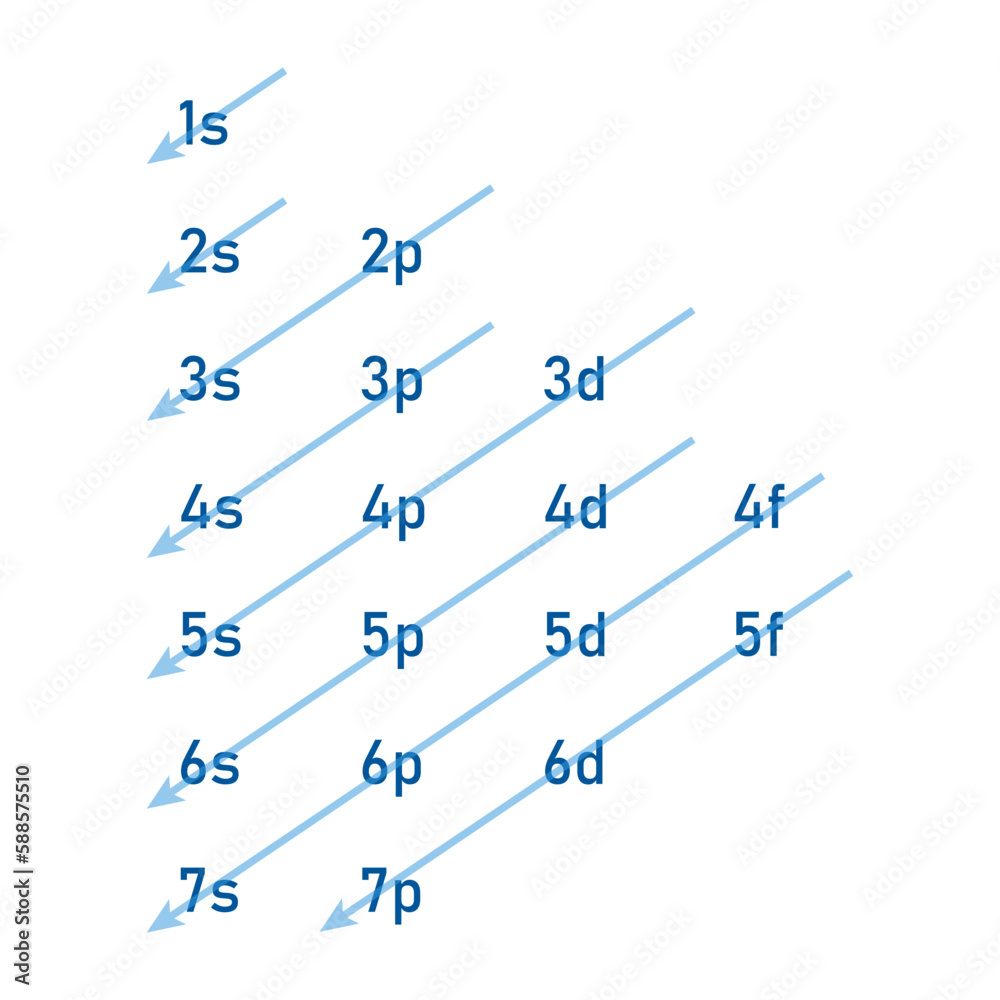
Typical order
1s2, 2s2 2p6, 3s2, 3p6 4s2, 3d10, 4p6, 5s2, 4d10, 5p6
Exceptions to orbitals
4s1, 3d5 instead of 4s2, 3d4
This gives us 2 half filled orbitals
Half full is slightly lower in energy that a fully filled orbital but there is still more stability
Same principle applies to copper
Atoms like to have the lowest energy as possible
Exceptions are usually when there is d4 or d9
Orbitals fill in order
Lowest energy to higher energy
Adding electrons can change the energy of the orbital
Full orbitals are the best solution
Half filled are next best
Makes them more stable and changes filling order
Ground vs Exited state
Excited state is when the orbitals do not full up fully before filling another one
Does not apply to exceptions as that is its ground state
Arborbs energy → less stable and often temporary
Ground state follows energy rules
Have the lowest possible energy
Metals
Electrical conductors
Have luster
Ductile (can be made into a wire)
Malleable
Non-metals
Generally brittle + non lustrous
Poor conductors of electricity and heat
Some are gasses (O, N, Cl)
Some are brittle solids (S)
one is a fuming dark red liquid (Br)
Metaloids
Border the 2 sides
Properties are intermediate between metals and non-metals
Very useful as they have both properties
Ex: silicon → tech used in electronics as it can carry a current but does not heat up
Group 1
Group 1: alkali metals
Forms a base (or alkali) when reacting with water
Only have 1 outer electron → more reactive
Group 2
Group 2: alkaline earth metals
Form bases with water, do not dissolve well, hence “earth metals”
Group 17
Group 17: halogens
Salt-forming
Half of diatomics (those that are more stable in 2) are halogens
Group 18
Group 18: noble gasses
Called inert gasses as they rarely take part in reactions → very stable
Have an electron configuration that has the outer p and s sublevels full
Groups 1,2,13-17
Wide range of properties → good representation
Some are metals, non-metals, and metaloids
Some are solids, while others are liquids or gasses
Their outer s and p electron configurations are not filled
Group 2-12
Groups 2-12: transition metals
Electron configurations have the outer s sublevel full and is not filling the d sublevel
A transition between the metal areas and non-metal area
Inner transitional metals
Inner transitional metals → located below the main body in horizontal rows
Electron configuration has outer s sublevel filled, and is now filling the f sublevel