CH101 Exam 2 Molecular Geometry
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Linear
What is the shape of this type of molecule?
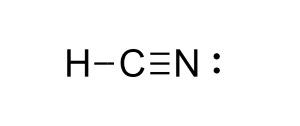
180
What is the bond angle for a linear molecule?
Trigonal planar (no lone pairs)
What is the shape of this molecule?
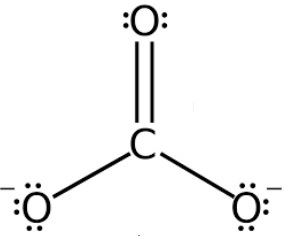
120
What is the bond angle for a trigonal planar molecule with no lone pairs?
Bent/angular (one lone pair)
What is the shape of this type of molecule?
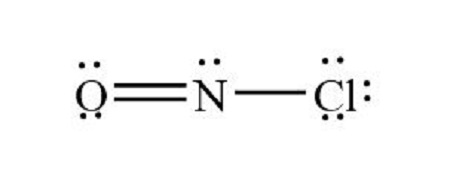
<120
What is the bond angle for a bent/angular molecule with one lone pair?
Tetrahedral
What is the shape of this type of molecule?
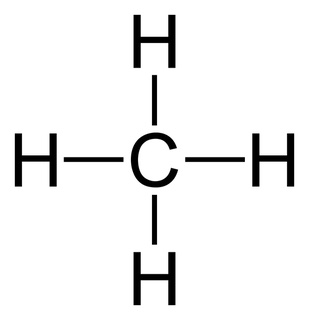
109
What is the bond angle of a tetrahedral molecule with no lone pairs?
Trigonal pyramidal
What is the shape of this type of molecule?
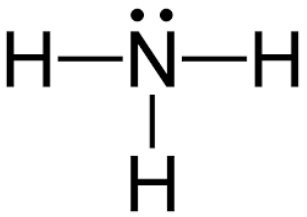
<109
What is the bond angle of a trigonal pyramidal molecule with one lone pair?
Bent/angular (2 lone pairs)
What shape is this type of molecule?
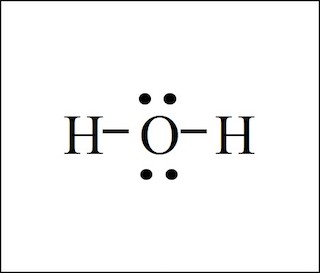
<<109
What is the bond angle of a bent/angular molecule with two lone pairs?