Physics HL Key concepts
1/199
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
200 Terms
velocity
rate of change of position with time
speed
rate of distance travelled along a path
acceleration
rate of change of velocity with time
translational equilibrium
a body in equilibrium has zero resultant force acting on it and therefore has zero acceleration
conservation of energy
The total energy of an isolated system can not be created or destroyed only converted from one form to the other.
Newton’s first law
A body at rest remains at rest, and a body in motion will remain in motion at a constant velocity unless acted upon by an unbalanced force.
Newton’s second law
The acceleration of an object depends on the mass of the object and the amount of force applied.
Newton’s third law
Whenever one object exerts a force on a second object, the second object exerts an equal and opposite force on the first.
Linear momentum
The product of a body’s mass and its velocity p =mv
Impulse
The change in momentum of a body. ∆p = mv - mu
law of conservation of momentum
If the total external force acting upon a system is zero, the momentum of the system is constant.
Work
Work is the ability to exert a force causing displacement of an object. W = fs
Power
The rate of work. P= work/time
Energy
Ability to do work
Kinetic energy
The energy an object has due to its translational motion.
Potential energy
Energy stored by a system due to the relative positions of its component parts. E = mgh (cannot be used for objects in space)
Elastic collision
A collision in which the total kinetic energy is conserved.
Inelastic collision
A collision in which some kinetic energy is transferred to other forms (eg internal energy, sound), therefore the total kinetic energy is less after the collision than before.
Gravitational field strength (g)
Force per unit mass experienced by a small point mass at the point. g = GM/r2
Weight
Force due to gravity
Universal law of gravitation
Every single point mass attracts every other point mass with a force that is directly proportional to the product of the masses and inversely proportional to the square of the radius. Gravitational force between two objects (F) = Gm1m2/r2
Test mass
A small mass which has a negligible effect on the gravitational field in which it is placed.
Uniform gravitational field
Where all field lines are always the same distance apart, almost exactly true close to the earths surface. Field is always in the same direction and magnitude.
Field lines
The strength of the field decreases as the lines get further apart as what happens when you get further from Earth.
Gravitational potential energy
The work done to move a body from infinity to a point a gravitational field. Ep = -Gm1m2/r
Escape speed
Speed of object at the surface of a planet so that it will escape from the gravitational field and travel to infinity.
Keplers laws
Planets orbit the sun in elliptical paths
A line segment joining a planet and the Sun sweeps out equal areas during equal intervals of time.
The square of the orbital period is directly proportional to the cube of the semi-major axis of its orbit. T2 =kr3
Keplers second law in depth
A line segment joining a planet and the Sun sweeps out equal areas during equal intervals of time. As the orbiting object approaches what it is orbiting, its potential energy will convert into kinetic energy so its speed will increase. The orbiting body then covers an equal area in an equal amount of time.
Deriving keplers third law
Using Newton’s law of gravitation and circular motion: mω2r=GM1M2/r2 and ω=2π/T
T2/r3 = 4π2/GM
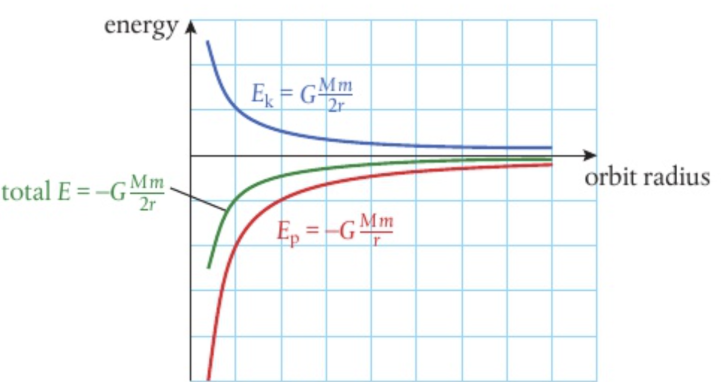
Energy of an orbiting body
Ek=GMm/2r = 1/2mv2
Ep= -GMm/r
total energy = Ep + Ek = -GMm/r + GMm/2r = -GMm/2r
Brownian motion
Any of various physical phenomena in which some quantity is constantly undergoing small, random fluctuations. Average value.
Temperature
Measure of the average kinetic energy of molecules, the natural flow of thermal energy is from high to low temperature.
Thermal energy
Thermal energy is the kinetic energy of the component particles of an object measured in jeaules.
Heat
Energy transferred from one body to another due to a temperature difference.
Thermal equilibirum
2 bodies that are in thermal contact are in thermal equilibrium when the net heat flow between them is zero, therefore the 2 bodies must have the same temperature.
Microscopic
On the scale of atoms and molecules (eg. particle mass, velocity, kinetic energy, momentum)
Macroscopic
On the scale of people (eg, temperature, volume, pressure, density)
Ideal gas
A gas that obeys the equation pV=nRT with the assumptions:
molecules are identical spheres of negligible volume (their total volume is much smaller than the volume of the container)
collisions are perfectly elastic
no forces between the molecules (except when they collide) so they move at constant velocity between collisions
Ideal gas assumptions
the collisions between molecules are perfectly elastic
the molecules are spheres
the molecules are identical
there are no forces between the molecules (except when they collide) so they have constant velocity between collisions
the molecules are very small, their total volume is much smaller than the volume of the container
Kinetic theory of gases
A model of the microscopic behvaior of gas particles that explains the macroscopic behavior of the gas.
Internal energy (U)
The sum of all random kinetic energies and mutual potential energies of the particles of the body or system. Does not include the kinetic and potential energy of the body as a whole. An ideal gas has no intermolecular forces (no mutual potential energies) therefore the ideal gas depends only on the kinetic energy of the particles.
Mole
Amount of substance of a system which contains as many elementary units as there are carbon atoms in 12×10-3 kg of Carbon-12. 6.02 × 10-23.
Molar mass
The mass of one mole of a substance.
Specific heat capacity
The amount of energy required to raise the temperature of unit mass through 1K.
Heat (thermal) capacity
The amount of energy required to raise the temperature of a substance through 1K.
Evaporation
The escape of molecules from the surface of the liquid.
Boiling
Occurs when molecules escape in the form of bubbles of vapour from the body of the liquid.
Specific latent heat
Energy per unit mass required to change the phase of a substance at its phase change temperature.
Pressure
The force per unit area exerted by something on the surface of a body. p = F/A
Indicator diagram
Graph of pressure against volume for agas
Isochoric (isovolumetric)
A process where the volume remains constant, therefore no work is done.
Isobaric
A process where the pressure remains constant
Isothermal
A process where the temperature remains constant, therefore the internal energy remains constant and change in U = 0
Adiabatic
A process in which there is no energy exchange between the system and surrounding during a compression or expansion of a gas. Work done is equal to the internal energy of the gas.
Entropy
Measure of disorder of a system
1st Law of Thermodynamics
Energy cannot be created or destroyed
2nd Law of Thermodynamics
In any process the overall entropy of the universe/a closed system increases.
Displacement (x) waves
Distance in a particular direction of a particle from its mean position
Amplitude (x0)
Magnitude of the maximum displacement from the equilibrium position.
Frequency (f)
Number of oscillations per unit time.
Period (T)
Time taken for one complete oscillation
Monochromatic
Single frequency/color
Simple harmonic motion
The motion that takes place when the acceleration of an object is always directed to the equilibrium position and is proportional to the displacement from a central position.
Damping
The process whereby energy is taken from the oscillating system (usually due to friction). Decreases the amplitude and there is a small decrease in resonant frequency.
Over-damped
The system tends to equilibrium slowly
Critically damped
The mass returns to the equilibrium position as quickly as possible without crossing it
Natural frequency
The frequency at which a system oscillates when disturbed from its equilibrium state.
Resonance
Maximum amplitude of oscilation when a periodic force is applied to it and the frequency of the force is equal to the natural frequency of vibration of the system.
Wavefront
Line joining neighbouring points that have the same phase/displacement
Ray
direction in which the wave energy is travelling
Travelling (progressive) wave
A wave that transfers energy between points in a medium.
Transverse wave
Motion of the particles is perpendicular to the direction of wave travel.
Longitudinal wave
Motion of the particles is parallel to direction of wave travel.
Wave frequency
The number of vibrations performed in each second by the source.
Wave period
The time for one complete vibration performed by the source.
Wavelength
Distance travelled by a wave during one oscillation of the source. (distance between successive crests or troughs)
Wave speed
Distance travelled per unit time by the energy of the wave (by a wavefront)
Refractive index
Ratio of speed in electromagnetic waves in vacuum to their speed in the medium.
Dispersion
Splitting/separation of white light into its component colours because different frequencies have different refractive indices.
Doppler effect
Change in received frequency of sound as a result of relative motion of source and observer.
Diffraction
Spreading out of light which occurs when the disturbance is of the same order of magnitude as the wavelength, and does not alter any properties of the wave.
Coherent waves
Two waves have the same frequency and a constant phase difference.
Principle of superposition
If two or more waves overlap the total displacement is the sum of the displacement of each wave. This can result in constructive and destructive interference.
Constructive interference
Occurs when occurs when the phase difference between the waves is an even multiple of π (nλ = path difference). Amplitude doubles
Destructive interference
Occurs when waves have the same amplitude in opposite directions so when they meet they completely cancel each other out. (n + 1/2)λ = path difference
Electric potential difference
Energy per unit charge to move positive test charge between points.
Electronvolt
The work done to move one electron through a potential difference of 1V.
Electric current (I)
The rate of flow of charge past a given cross-section of a conductor.
Resistance (R)
The ratio of potential difference across a load to current in the load.
Electromotive force (emf)
The power supplied per unit current or work done per unit charge in moving charge completely around the circuit.
Ohm’s law
The resistance of a conductor is constant and the current is proportional to the potential difference if its temperature is constant.
Electric field strength
The force per unit charge felt by a positive test charge placed in the field.
Electric potential
The work done per unit charge in bringing a small positive charge from infinity to that point.
Magnetic flux
The number of magnetic field lines passing trhough the region.
Magnetic flux linkage
Product of number of turns in a coil and the flux through the coil.
Faraday’s law of electromagnetic induction
emf induced is proportional to the rate of change of flux linking the coil.
Lenz’s law
The induced emf is in such a direction that its effect is to oppose the change causing it.
rms voltage
The value of the direct voltage that gives the same power dissipation as the average ac power dissipation.
Direct current (DC)
Occurs when the current flows in one constant direction.
Alternating current (AC)
Occurs when the electric current periodically inverts its direction.