Chemical Stability
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Define Stability.
the chemical and physical integrity of the dosage unit and, when appropriate, the ability of the dosage unit to maintain protection against microbiological contamination
ALSO
the extent to which a product retains, within specified limits, and throughout its period of storage and use (i.e. its shelf life), the same properties and characteristics that it possessed at the time of manufacture”.
Define Shelf life.
Time from date of manufacture that a drug product will remain in its approved product specifications while stored under defined conditions.
time lapse from initial preparation to the specified expiration date
Where can you find the shelf life of a dosage form?
The monograph specifies identity, strength, quality, and purity throughout the shelf life of the product
USP monographs state percent ranges for labeled strengths of active ingredients, where the lower number is the shelf life value
What is BUD?
Date after which an article shall not be used.
How do we find BUD?
Unless otherwise specified in the individual monograph, for non-sterile solid/liquid products repackaged into unit-dose or single unit containers the Beyond-use date shall be no later than
(a) the expiration date on the manufacturer’s container, or
(b) 1 year from the date the drug is dispensed, whichever is earlier.
Chemical Stability
Each active ingredient retains its chemical integrity and labeled potency, within the specified limits.
THROUGHOUT THE SHELF LIFE
Physical Stability
The original physical properties, including appearance, palatability, uniformity, dissolution, and suspendability, are retained.
THROUGHOUT THE SHELF LIFE
Microbiological Stability
Sterility or resistance to microbial growth is retained according to the specified requirements. Antimicrobial agents that are present retain effectiveness within the specified limits.
THROUGHOUT THE SHELF LIFE
Therapeutic Stability
The therapeutic effect remains unchanged.
THROUGHOUT THE SHELF LIFE
Toxicological Stability
No significant increase in toxicity occurs.
THROUGHOUT THE SHELF LIFE
Factors that can impact product stability:
Temp → High temp = Inc chemical degradation
pH → influences rate of drug decomposition
Moisture → can catalyze chemical rxns & Promotes microbial growth
Light → Energy/thermal effect = OXIDATION
Dosage form → Solid > Liquid
Drug Incompatibility →
Oxygen
How do we prevent drug container interactions?
Non-PVC tubing minimizes leaching
Contact of undiluted concentrate with plasticized PVC equipment or devices is NOT recommended
Diluted TAXOL solutions should be stored in bottles or plastic bags and administered through polyethylene lined administration sets
Preconditioned tubing for insulin
Elements for establishing an EXPIRATION date for a drug product
Characteristics of the drug
Active drug content
Degradants
Characteristics of the dosage form
– Appearance, color, pH, odor, coating integrity
– Moisture content, content uniformity
– Friability (tablets)/brittleness (capsules), tablet hardness
– Dissolution characteristics
– Viscosity (for liquids)
– Particle size eg suspensions and emulsions
– Emulsion phase separation – creams and liquids.
– Lack of microbial contaminations (creams, liquids, etc
What are the chemical routes of degradation?
Hydrolysis
Oxidation
Photodegradation
Drug-drug or Drug-Excipient incompatibilities
What is hydrolysis?
Breaking of molecular bonds by reaction with water
Most common cause of chemical degradation since drugs formulated in aqueous media or will be exposed to water during dissolution
Esters, Amides, Lactams, Lactones
Catalysts of Hydrolysis
• Presence of water
• pH
• Presence of general acids and bases used as buffers/salts (citrate, acetate, phosphate)
• Temperature
Hydrolysis rate can be reduced by:
• Temperature (0-5 °C)
Humidity <40%
• Desiccant in packaging
Multi-layered tablets
• Film coating
Buffers for solutions
• Suspensions for liquids
What is Oxidation?
Increase in the number of C-O bonds in a molecule (loss of electrons) or a reduction in the number of C-H bonds
Catalyzed by trace metals, light, heat
Can lead to:
Hydroxylation
Dehydrogenation
Carboxylation
Deamination
Demethylation
Most rxns involve free radicals (unpaired electrons)
Initiation, propagation, termination
Drugs Susceptible to Oxidation
Catechols → Methyldopa, Epi
Thiol;s → 6-Mercaptopurine, Captopril
Phenothiazines → Promethazine
Polyunsaturated molecules → Vit A, Ergocalciferol, cholecalciferol
Oxidation Catalysts
• Cu+2, Fe+2,+3 ions - Trace amounts of heavy metals
• Sunlight and fluorescent light (UV from both)
• O2, oxidizing agents
• Heat
Oxidation can be reduced by:
–Nitrogen, carbon dioxide, to replace air in container
–Temperature 0-5 °C
– Protect from light
–Chelating agents – EDTA (ethylene diamine tetracetic acid
What do Antioxidants do?
They interrupt the propagation by interacting with the free radical
Suicide substrate - more susceptible to oxidation and are preferentially oxidized to consume the available oxygen
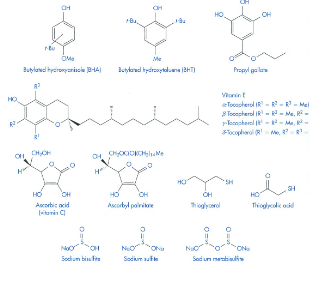
Drug-Drug/Drug-Excipient Incompatibilities
Amines: Many drugs contain the amino (-NH2) functional group which is highly reactive with a number of other chemicals. Examples include:
Carboxylic acid
Carboxylate Ester
Large Anions
Aldehyde
• Esters: Beside reacting with amines, esters can also react with alcohols to undergo an alcoholysis reaction (Transesterfication).
– Formulation of aspirin (an ester) in polyethylene glycol or the preparation of an aspirin with either morphine or codeine (contain alc.)