Endoplasmic Reticulum and Disease
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
what is the endoplasmic reticulum?
serves as the cell's biosynthetic powerhouse and a highway for the transport of molecular materials
a network of membranous tubules and flattened sacs that extend throughout the cytoplasm
what are the two types of endoplasmic reticulum?
rough and smooth
what are the functions of the ER?
Protein synthesis (RER)
• Lipid metabolism (SER)
• Calcium storage (SER)
• Drug detoxification (SER)
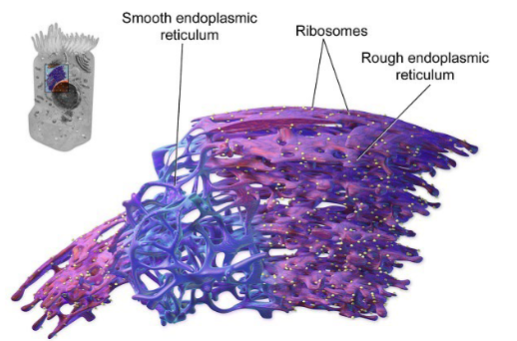
what is the rough endoplasmic reticulum?
characterized by the presence of ribosomes on its cytoplasmic surface.
The site of synthesis for secretory, membrane-bound, and organelle-targeted proteins
Newly synthesized proteins enter the RER lumen where they undergo folding and post-translational modifications.
what is the smooth endoplasmic reticulum?
lacks ribosomes and has a more tubular appearance.
Involved in lipid and steroid hormone synthesis, crucial for cell membrane formation and signalling.
• The SER also plays a role in detoxifying metabolic by-products and xenobiotics.
• It regulates intracellular Ca²⁺ levels, important for muscle contractions and other signalling pathways.
how are proteins manufactured in the rough ER?
Ribosomes translate mRNA into polypeptide chains that are co- translationally translocated into the RER.
• Inside the RER, proteins are folded with the help of chaperones.
• They undergo modifications such as glycosylation and disulphide bond formation.
Properly folded proteins are packaged into vesicles for transport to Golgi apparatus
what are the defining characteristics of type I membrane protein insertion into the ER?
Single transmembrane domain
• N-terminus in the ER lumen, C-terminus in the cytosol
• Contains a cleavable N-terminal signal sequence
what is the insertion process for type I membrane protein insertion into the ER?
Begins with ribosome translation of the signal sequence
Signal Recognition Particle (SRP) pauses translation and directs the ribosome to ER
Signal peptide is inserted into the translocon, and cleaved off as translation resumes
The hydrophobic stop-transfer sequence halts translocation, anchoring the
protein in the membrane
The C-terminal continues to be synthesized into the cytosol
what are two protein examples of type I membrane protein insertion into the ER?
Glycophorin
CD4
what are the defining characteristics of type II membrane protein insertion into the ER?
Single transmembrane domain
• N-terminus in the cytosol, C-terminus in the ER lumen
• Contains a signal-anchor sequence not cleaved
what is the insertion process for type II membrane protein insertion into the ER?
Begins with ribosome translation of internal signal-anchor sequence
• SRP directs the ribosome to the ER; signal-anchor sequence initiates insertion
• The orientation is dictated by positive charges flanking the signal-anchor sequence
• The polypeptide chain grows into the ER lumen, forming the C-terminus
what are the two example proteins for type II membrane protein insertion into the ER?
Asialoglycoprotein receptor
• G protein-coupled receptors (some types)
what are the defining characteristics for type III membrane protein insertion into the ER?
Single transmembrane domain
• N-terminus in the ER lumen, C-terminus in the cytosol
• Signal-anchor sequence remains as a transmembrane segment
what is the insertion process of type II membrane protein insertion into the ER?
Translation begins with an internal signal-anchor sequence
• SRP binds to the signal-anchor and guides the complex to the ER
• The orientation is generally N-lumenal, often influenced by the distribution of
positive charges
• Protein continues to elongate, inserting the C-terminal into the cytosol
what are example proteins of type III membrane protein insertion into the ER?
Cytochrome P450 enzymes
• Flippase proteins
what are the defining characteristics for type IV membrane proteins: multipass insertion?
Multiple transmembrane domains
• Complex orientation with both termini on the same or opposite sides of the ER
membrane
• Contains several signal-anchor and stop-transfer sequences
what is the insertion process for type IV membrane proteins: multipass insertion?
Translation begins with a signal-anchor sequence that is not cleaved
• Multiple signal-anchor and stop-transfer sequences guide the ribosome in
inserting the protein
• The protein loops in and out of the translocon, inserting multiple domains into the
membrane
what are example proteins of type IV membrane proteins: multipass insertion?
G protein-coupled receptors with multiple transmembrane domains
• Ion channels like the potassium channel
what is translocation?
Proteins synthesized in the ER are destined for various locations: lysosomes,
endosomes, or the plasma membrane.
Transport to these locations is highly regulated and critical for proper cellular function
what is vesicle transport?
Vesicles are the primary mode of transport for proteins from the ER to their
destinations.
Bud from the ER or Golgi apparatus, vesicles ferry encapsulated proteins through the cytoplasm
what are sorting signals?
Proteins contain specific amino acid sequences that act as postal codes, directing them to the correct cellular address.
These signals are recognized by adapter proteins which mediate the sorting of proteins into vesicles
what is the post-transitional pathway?
Proteins are synthesized in the ER, processed in the Golgi, and then transported out of the cell.
• Secretory proteins are packed into vesicles that bud from the Golgi and migrate
towards the plasma membrane
what is constitutive secretion?
Continuous, non-selective process where secretory vesicles fuse with the
plasma membrane to release their contents.
Operates constantly in all cells, delivering proteins like extracellular matrix components.
what is regulated secretion?
Selective, triggered process in response to specific signals or environmental
cues.
• Common in cells that produce hormones, neurotransmitters, and digestive
enzymes.
what is the import mechanism of protein import into the er?
Proteins destined for the ER have a signal sequence that directs them to the ER membrane.
The signal recognition particle (SRP) binds to this sequence and pauses translation.
The SRP-ribosome complex docks on the ER membrane, and the protein is threaded into the ER lumen through a translocon channel
what is protein folding?
Once inside the ER, proteins must fold into their three-dimensional shapes to become functional.
The ER provides an optimized environment for protein folding, with a unique set of enzymes and conditions
what are chaperones and foldases?
Molecular chaperones, such as BiP, assist in proper protein folding and prevent aggregation.
Foldases, like protein disulphide isomerase (PDI), facilitate the formation of disulfide bonds between cysteines
what are post-transitional modifications?
Proteins in the ER are modified through processes like glycosylation, which attaches sugar molecules to specific amino acids.
• Other modifications include the formation of disulfide bonds and proper folding to achieve mature protein conformation.
what is ER-associated degradation?
A surveillance system that identifies and disposes of misfolded or unassembled proteins.
• Misfolded proteins are retrotranslocated back into the cytosol, ubiquitinated, and targeted for degradation by the proteasome
what is an unfolded protein response?
A cellular stress response triggered by the accumulation of unfolded proteins
in the ER.
UPR aims to restore normal function by halting protein translation, degrading misfolded proteins, and activating the signaling pathways that increase the production of molecular chaperones.
what are the 3 key UPR signal activator proteins?
Inositol requiring 1 (IRE1)
• PKR-like ER-kinase (PERK)
• Activating factor 6 (ATF6)
what are the 3 domains in UPR signalling?
ER luminal domain (LD)
• Single pass membrane spanning domain
• Cytosolic domain
what is UPR signalling?
Adaptive unfolded protein response (UPR) signalling under acute endoplasmic reticulum (ER) stress.
Accumulation of unfolded protein triggers UPR by activation of
(B) PERK
(C) Activating Transcription Factor 6.
(A) inositol-requiring 1 (IRE1)
This leads to upregulation of ER-associated degradation protein and folding chaperons to mitigate ER stress and maintain homeostasis
what are clinical implications of UPR signalling?
Persistent ER stress and an overwhelmed UPR can contribute to the development of diseases like neurodegeneration, diabetes, and cancer.
Therapeutic strategies targeting ER stress pathways are being explored to treat these conditions
what is defective protein folding?
Misfolding can occur due to genetic mutations, environmental factors, or a combination of both, leading to loss of function or toxic gain of function.
Misfolded proteins can aggregate, leading to cellular stress and activation of the UPR, which may result in apoptosis if homeostasis cannot be restored.
what causes cystic fibrosis?
Caused by mutations in the CFTR gene leading to misfolded CFTR protein, which results in faulty chloride ion transport
what causes alpha-1 antitrypsin deficiency?
Due to mutations in the SERPINA1 gene, misfolded alpha-1 antitrypsin accumulates in the liver, impairing lung function due to unregulated elastase activity
what are pharmacological chaperones?
Small molecules that stabilize the native state of proteins, improving their folding and trafficking
what are proteostasis regulators?
Compounds that modulate the UPR pathways, chaperone levels, and proteasomal degradation to alleviate stress
what is gene therapy?
Strategies to replace defective genes or introduce correct copies to restore normal protein function
what is Alpha-1 antitrypsin?
AAT is a protease inhibitor produced in the liver, functioning primarily to protect the lungs by inhibiting neutrophil elastase.
It is synthesized as a single polypeptide chain that folds into a stable tertiary structure within the ER of hepatocytes
what is the pathogenesis of AAT deficiency?
Caused by mutations in the SERPINA1 gene, leading to the production of a misfolded variant of AAT called Z-AAT.
Misfolded Z-AAT accumulates in the ER of hepatocytes, forming insoluble polymers that cause liver cell damage and ER stress.
what are symptoms of AAT deficiency?
Liver damage due to ER stress and apoptosis of hepatocytes.
Reduced levels of functional AAT in the blood lead to unchecked neutrophil elastase activity, resulting in lung tissue damage and emphysema
what are therapeutic interventions of AAT deficiency?
Augmentation Therapy: Infusion of purified AAT to restore its protective levels in the lungs.
Small Molecule Correctors: Compounds that assist in proper folding and prevent polymerization of Z-AAT.
Gene Therapy: Approaches to correct the underlying genetic defect, providing a source of functional AAT.