Evolutionary Medicine Exam 2
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
86 Terms
Why are mosquitoes most deadly animal
Because they are vectors
Vector
living organisms that can transmit infectious pathogens between animals (including humans)
Many are bloodsucking insects
What can be transmitted through vectors
Parasites, bacteria or viruses
What challenges are presented to pathogens that are transmitted via vectors
They need to adapt to both vector and host
(higher burden in tropical, subtropical areas) (affects poorest populations)
(finances would greatly affect the outcomes of a sick person)
Amplifying host:
an animal in which a pathogen can efficiently replicate itself and that can spread the pathogen to a vector
Dead-end host:
an animal infected by a vector-borne pathogen but that cannot spread the pathogen to a vector
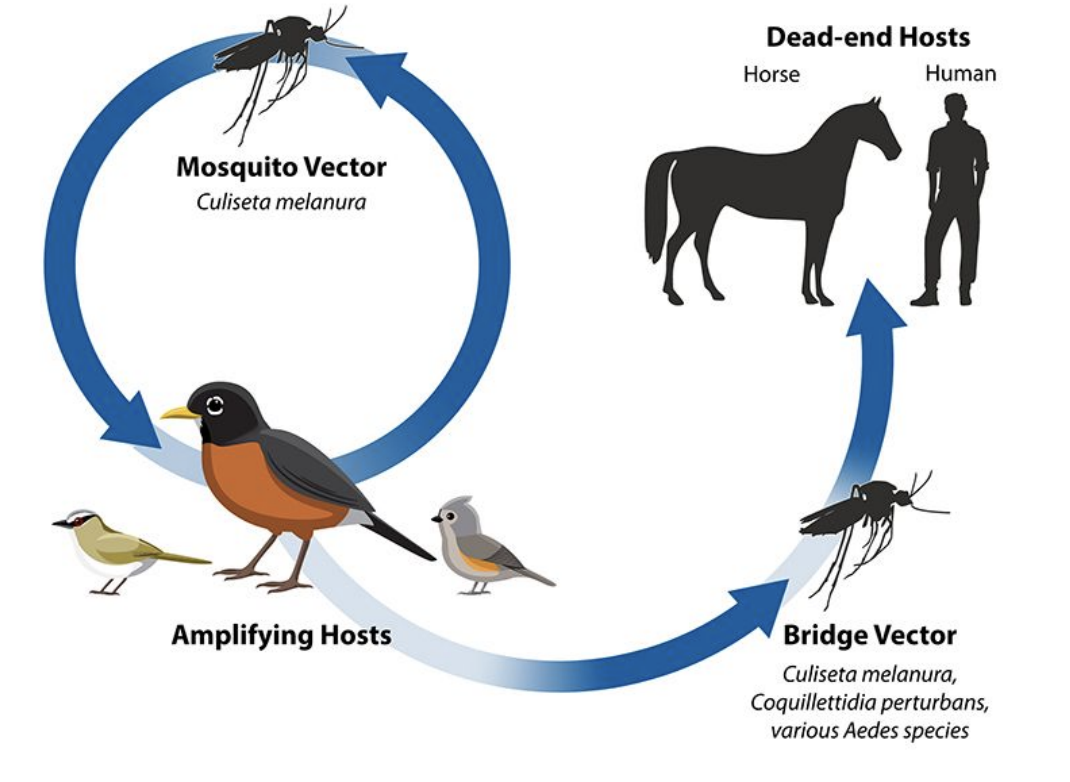
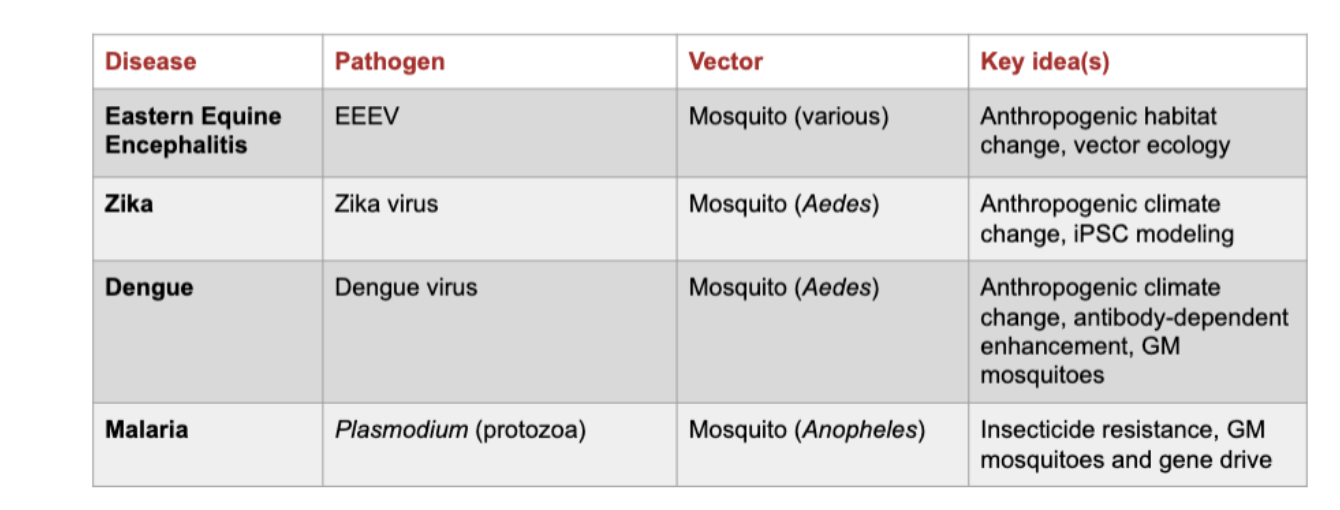
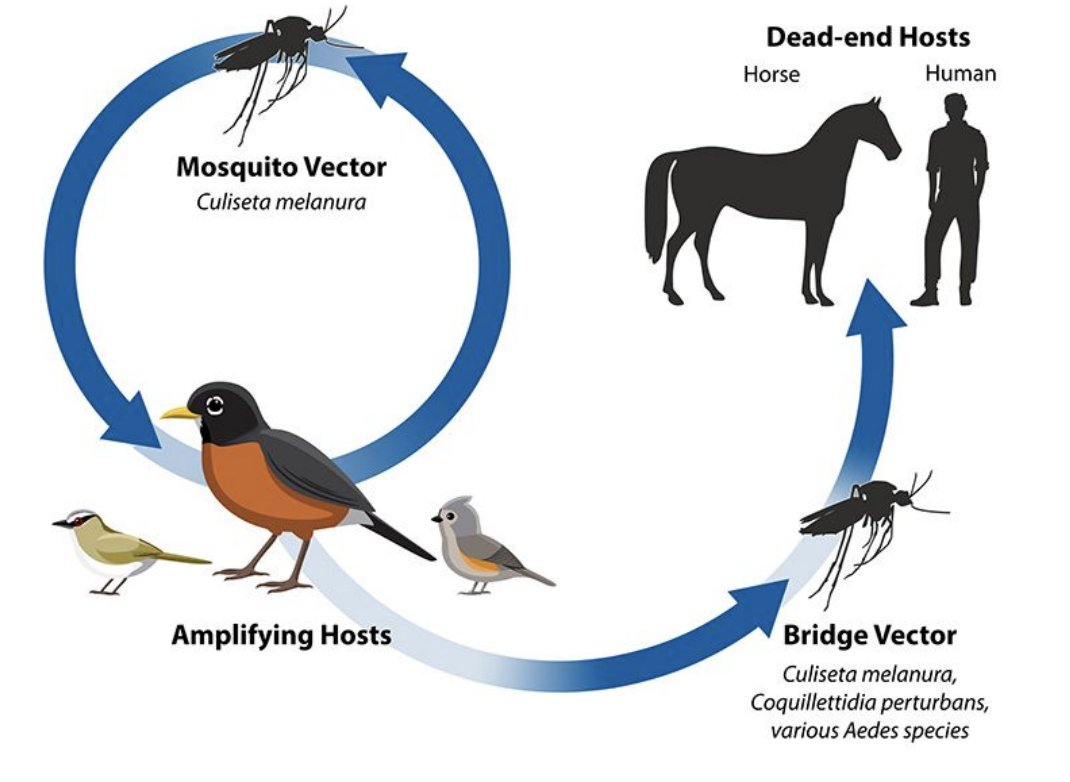
What is the transmission cycle of Eastern Equine Encephalitis (EEE)?
The EEE virus cycles between Culiseta melanura mosquitoes and birds (amplifying hosts).
Bridge vectors can transmit the virus from infected birds to humans or horses, which are dead-end hosts because they don’t develop enough viremia to infect mosquitoes.
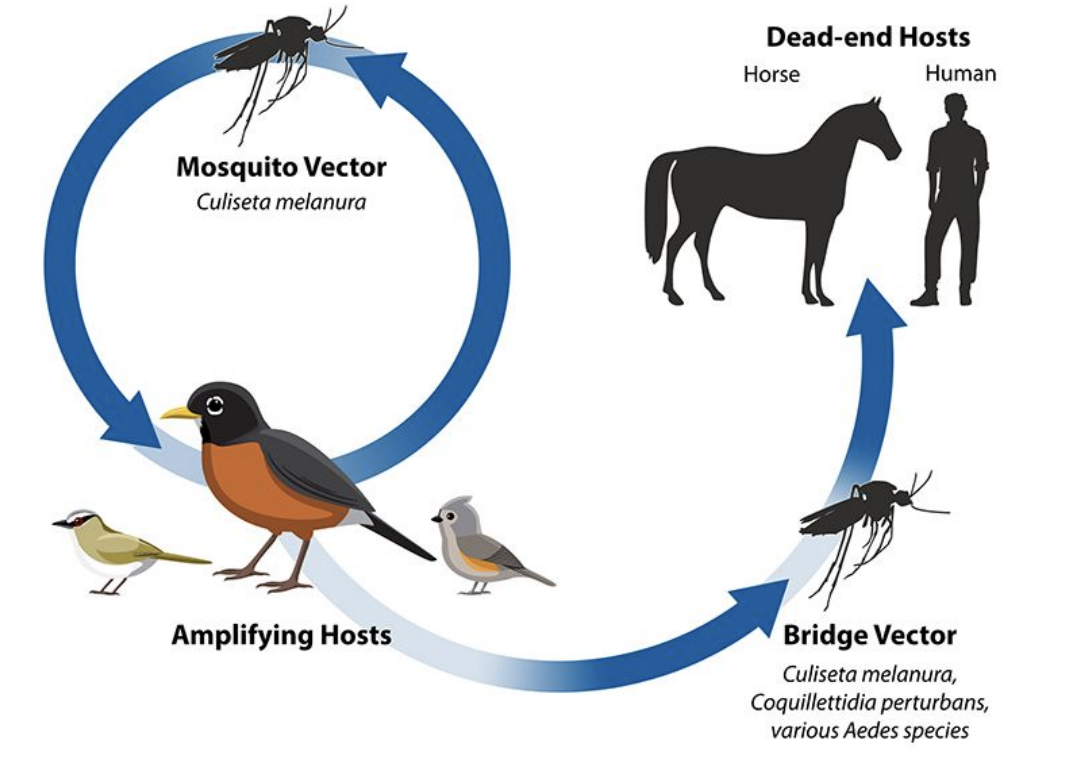
Why are humans and horses considered dead-end hosts in Eastern Equine Encephalitis?
They can become infected through mosquito bites but cannot spread the virus back to mosquitoes since viral levels in their blood are too low for transmission
How do environmental changes affect Eastern Equine Encephalitis transmission?
Deforestation initially reduced EEE cases by removing bird habitats
Wetland and forest restoration reintroduced bird hosts (e.g., robins), allowing virus amplification
Illustrates the link between anthropogenic habitat change and vector ecology
How can Eastern Equine Encephalitis persist seasonally?
The virus can survive winter conditions in mosquito populations, enabling seasonal reemergence when mosquitoes become active again.
Where did Zika originate?
→ Uganda (Zika Forest, 1947); later spread to Asia, Pacific Islands, and Brazil (2015 outbreak).
Why was a Zika outbreak alarming?
Linked to microcephaly in newborns — babies born with smaller brains.
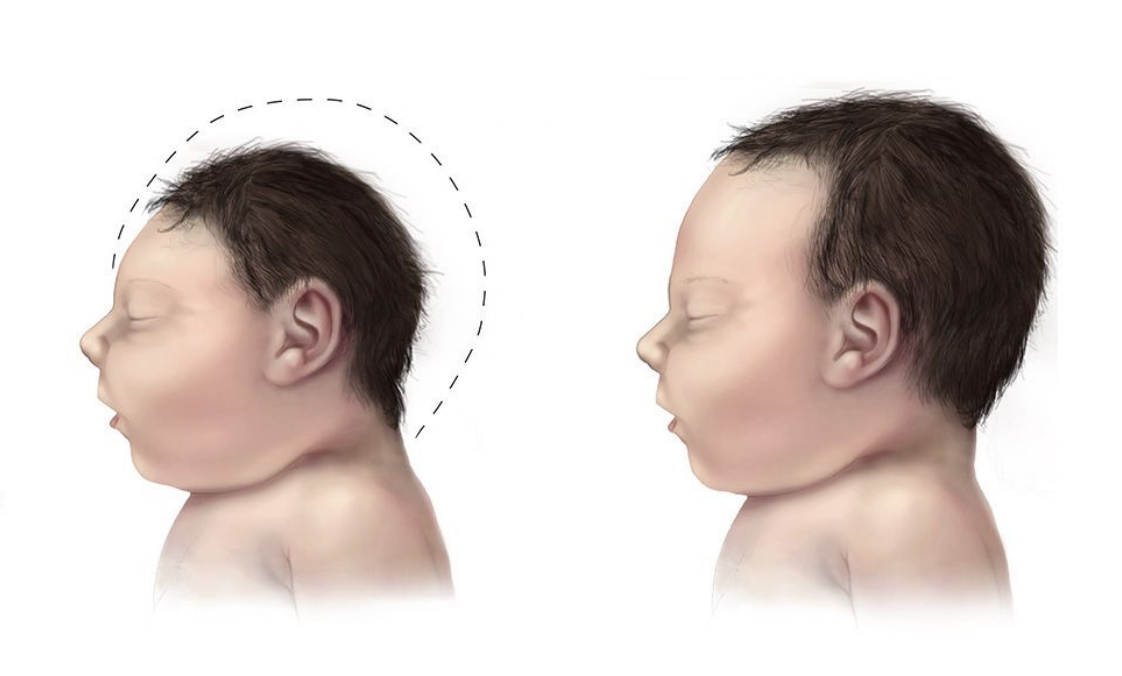
How did scientists figure out Zika causes microcephaly?
Infected human brain organoids (mini-brains) → saw impaired growth and cell death (Garcez et al., 2016).
How is Zika transmitted?
Mainly by Aedes mosquitoes, but also sexually and from mother to fetus.
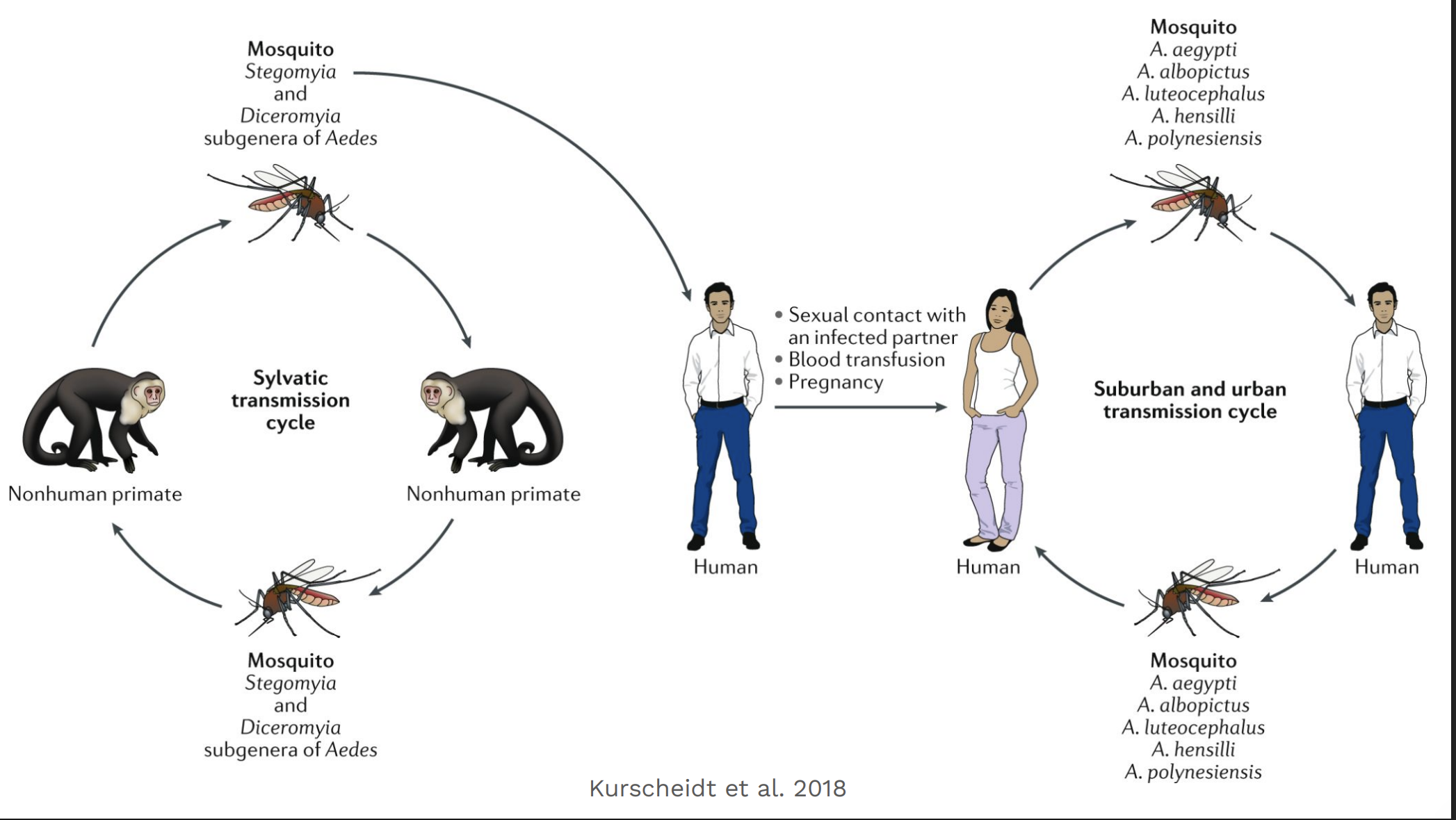
Why are people in the U.S. worried?
The Aedes mosquitoes that carry Zika live in parts of the U.S., so local spread is possible.
What causes dengue?
Dengue virus, a flavivirus spread by Aedes mosquitoes (A. aegypti and A. albopictus).
Why is dengue so widespread?
3 billion people (40% of world’s population) live in areas where dengue is endemic — mostly in tropical/subtropical regions (see red areas on map).
Why is it hard to make a vaccine against dengue?
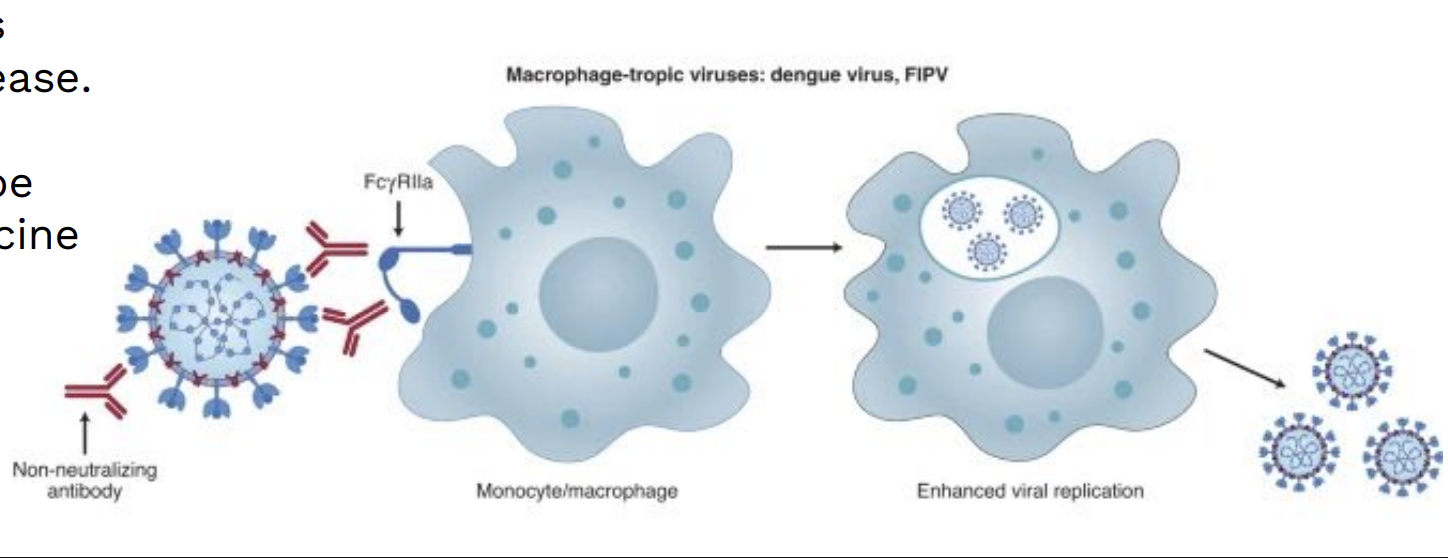
Dengue has 4 serotypes (DENV 1–4). Infection gives lifelong immunity to one, but only partial, cross-reactive immunity to others.
If a person is later infected with a different serotype (or after vaccination), non-neutralizing antibodies can help the virus enter immune cells — this is antibody-dependent enhancement (ADE) — leading to higher viral loads and more severe disease.
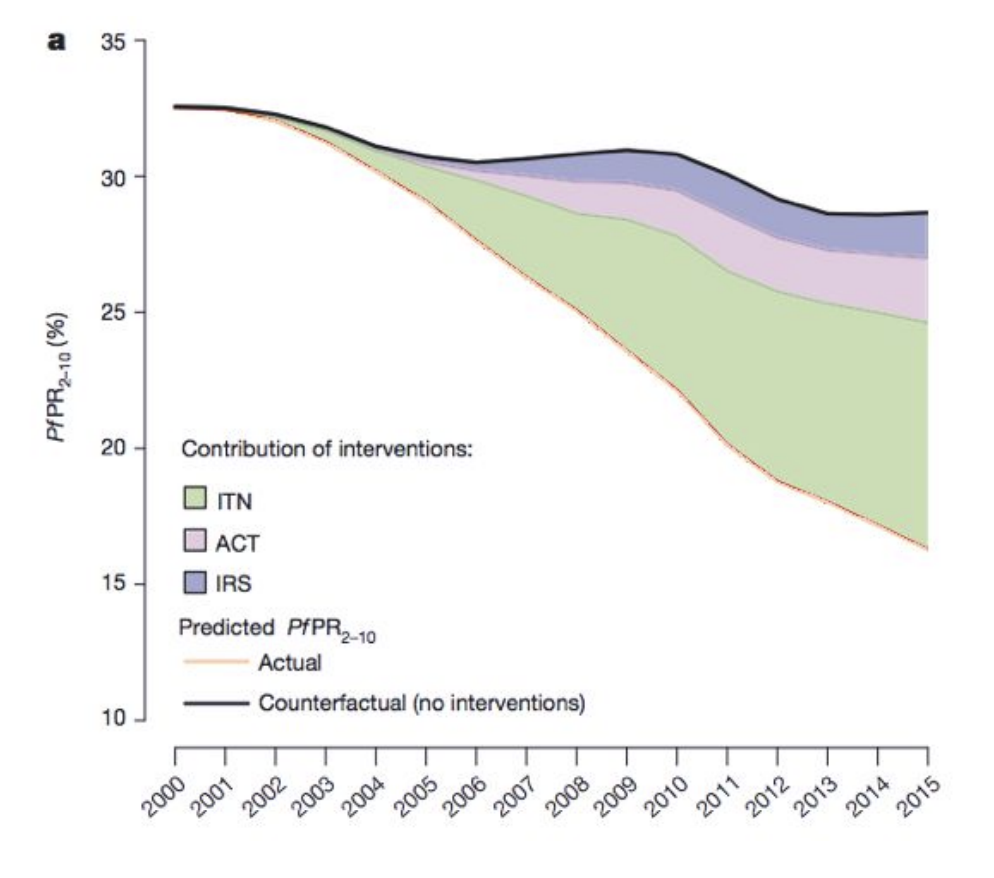
What intervention has saved the most childhood lives from malaria?
Insecticide-treated bednets (ITNs) — single greatest reduction in malaria burden.
Why are Insecticide-treated bednets (ITNs) less effective now? (malaria)
Mosquitoes evolved insecticide resistance, reducing ITN efficacy.
Selective sweeps show resistance variants rising rapidly in mosquito populations.
What is a promising new malaria control method?
Genetically modified (GM) mosquitoes — do not rely on insecticides.
Population suppression → engineered mosquitoes cause population crash (offspring die).
Population replacement → engineered mosquitoes outcompete wild ones, can’t transmit malaria.
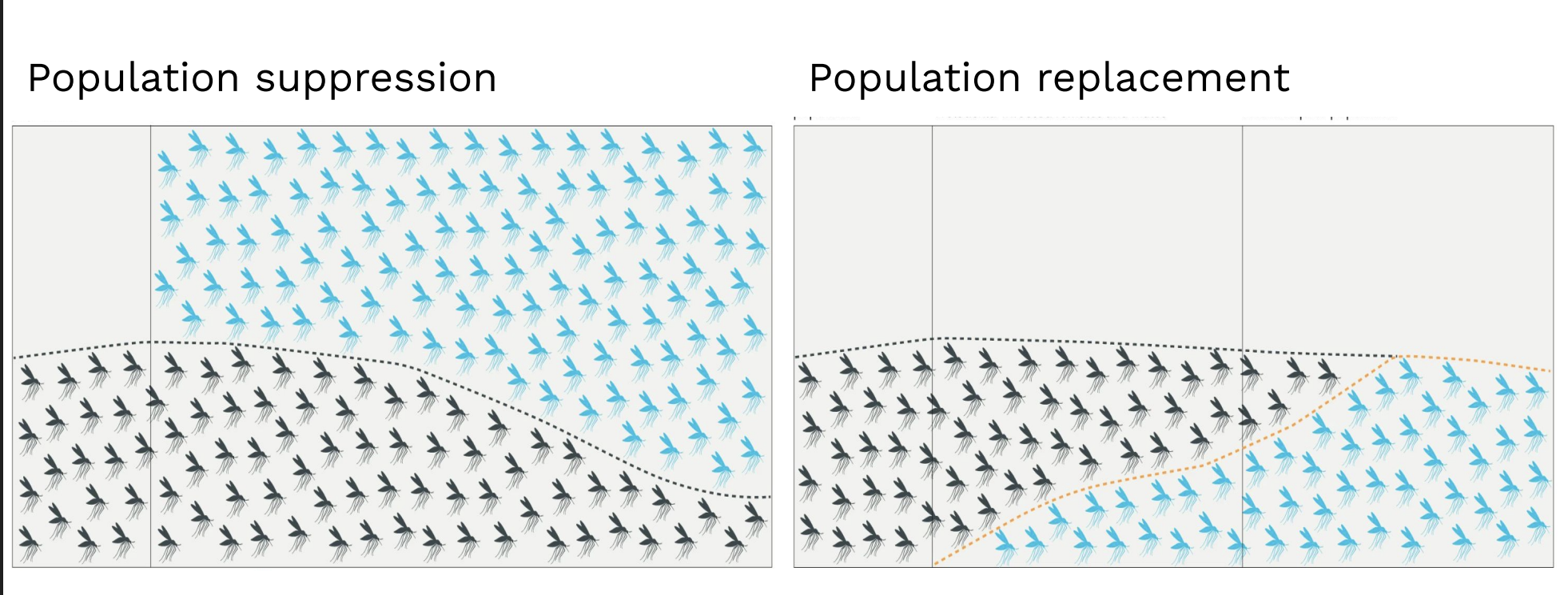
Which GM strategy for malaria is riskier for the environment?
Population replacement — because it permanently alters the ecosystem’s gene pool, while suppression only reduces numbers.
What is the goal of a vaccine?
To train the immune system to recognize and fight a pathogen without causing severe illness.
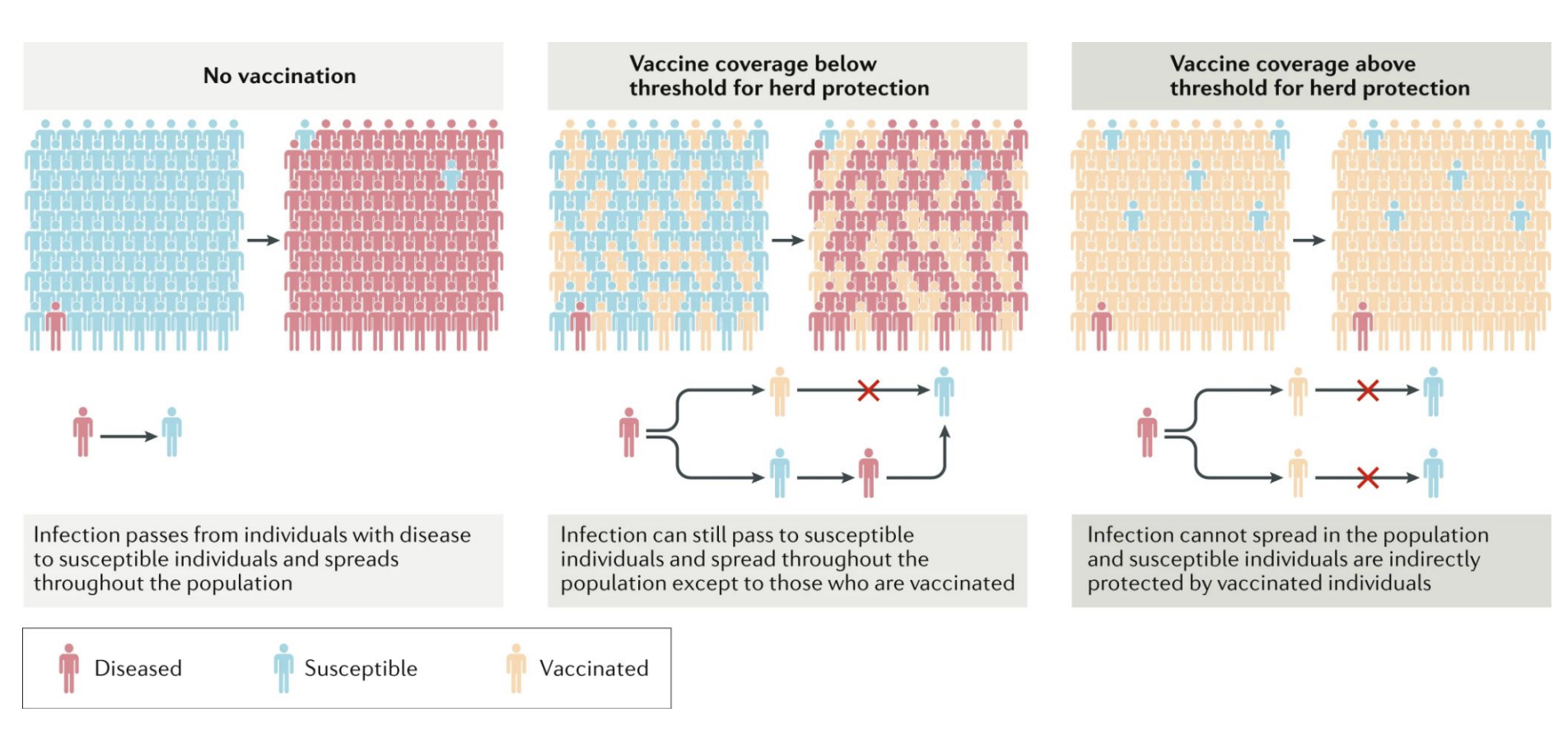
Herd immunity: When enough of a population is immune (by vaccination or infection), disease spread slows or stops, protecting those who are unvaccinated or immunocompromised
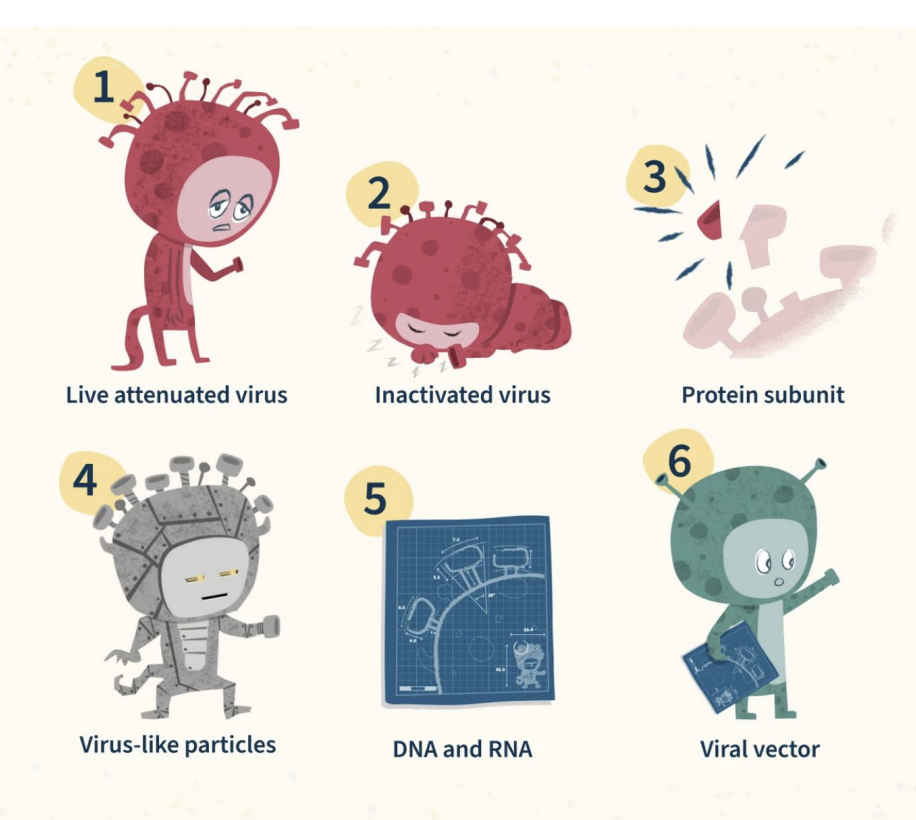
What are the six main approaches to a vaccine?
Live attenuated virus – weakened by mutation; replicates without causing disease.
Inactivated virus – killed by heat, radiation, or chemicals.
Protein subunit – contains just a viral antigen (e.g., spike protein).
Virus-like particles (VLPs) – mimic virus structure but contain no genome.
DNA/RNA vaccines – encode viral protein; host cells make antigen (e.g., mRNA COVID-19).
Viral vector vaccines – use harmless virus (e.g., adenovirus) to deliver viral genes.
How do mRNA and viral vector vaccines differ?
mRNA vaccine: delivers messenger RNA directly into host cells → translation into viral protein → immune response.
Viral vector vaccine: uses a non-replicating virus (like adenovirus) as a carrier to deliver DNA encoding the viral antigen.
Who developed the smallpox vaccine?
Edward Jenner (1796) — observed that milkmaids with cowpox were protected from smallpox.
He inoculated a boy with cowpox, then exposed him to smallpox → boy did not get sick.
Others in earlier cultures (China, India, Africa, Ottoman Empire) also practiced forms of inoculation before Jenner.
What virus was used for the smallpox vaccine?
Vaccinia virus (VACV) — a poxvirus related to cowpox and horsepox, not actually smallpox itself.
Provides cross-protection against Variola virus (smallpox).
What is the significance of smallpox eradication?
First and only human disease eradicated (declared by WHO in 1977).
Achieved through global vaccination campaigns and ring vaccination strategy.
What causes Lyme disease and how is it transmitted?
Caused by the bacterium Borrelia burgdorferi, a spirochete.
→ Transmitted by the black-legged tick (Ixodes scapularis).
Vector: Deer tick
Reservoir host: White-footed mouse (Peromyscus leucopus)
Deer support adult tick stage (not main reservoir).
Which tick stage most commonly transmits Lyme disease to humans?
Nymph stage
Nymphs are small and easily missed.
More likely to feed long enough to transmit infection.
Describe the life cycle of the tick.
wo-year cycle:
Egg → Larva → Nymph → Adult
Larvae feed on small animals (like mice).
Nymphs infect humans.
Adults feed on deer, then lay eggs.
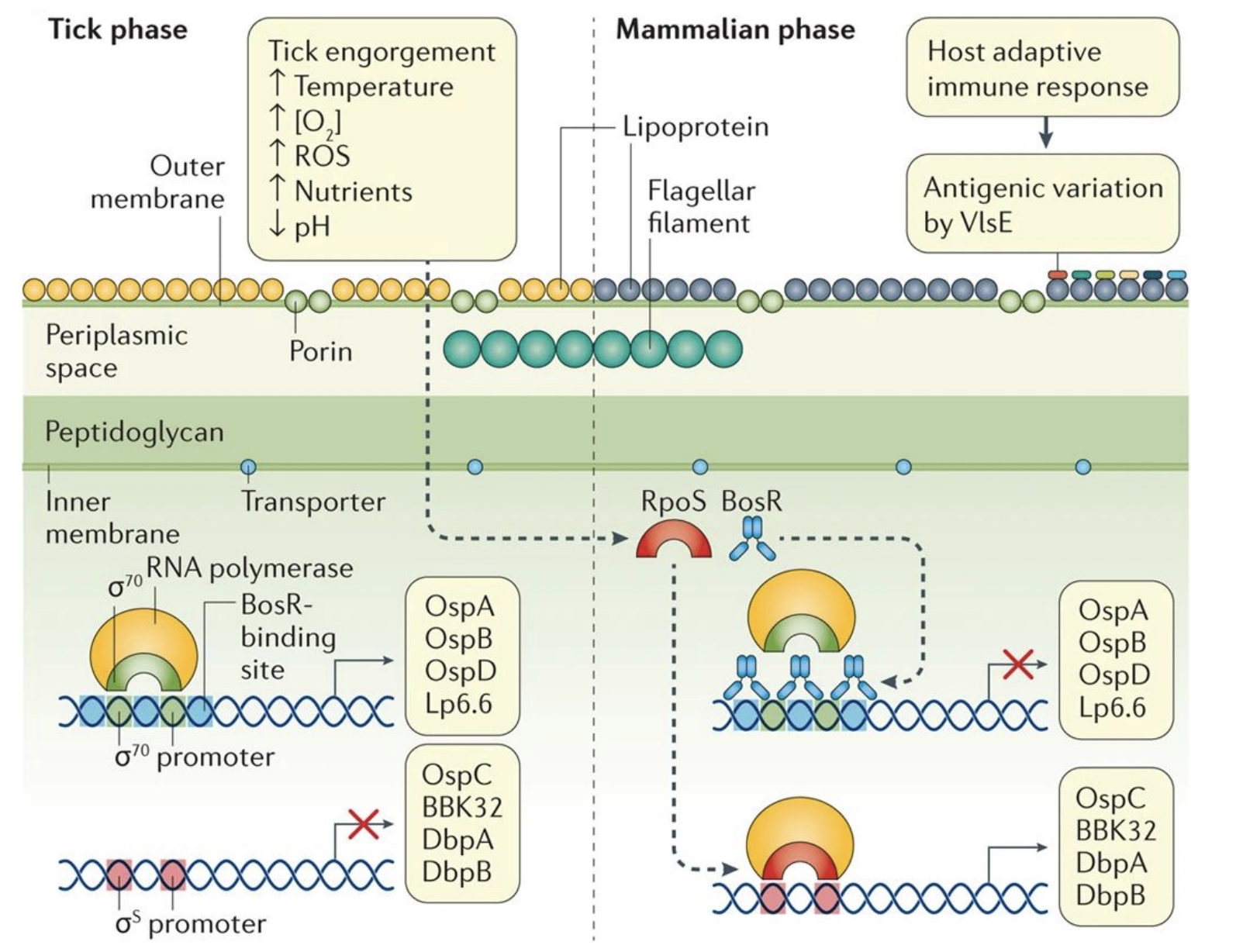
What makes Borrelia burgdorferi unusual?
Must survive in both tick and mammalian hosts → major gene expression changes.
Tick phase: outer membrane proteins (OspA, OspB).
Mammalian phase: different proteins (OspC, BBK32, DbpA/B).
Triggered by ↑temperature, ↓O₂, ↑nutrients, ↓pH during tick feeding.
What was the Lyme vaccine and why was it discontinued?
Vaccine: LYMErix (1998–2002) – recombinant OspA protein vaccine.
Showed ~76% efficacy after 3 doses, safe and effective.
Withdrawn due to low demand and public concern about side effects (not scientifically supported).
What’s a new vaccine approach for Lyme disease?
Target the tick instead of the bacterium 🦟
“Stop the tick” strategy: aims to block tick feeding or pathogen transmission.
Dogs have 4 safe Lyme vaccines, humans currently have none.
Why is malaria vaccine development so difficult, and what are the main vaccine targets?
Because Plasmodium falciparum is a complex eukaryotic parasite with multiple life stages (in both mosquito and human), each expressing different antigens, making immune targeting difficult.
Main vaccine strategies target different stages of the life cycle:
Pre-erythrocytic vaccines (e.g., RTS,S) → target sporozoite/liver stage.
Blood-stage vaccines → target parasite replication in RBCs.
Transmission-blocking vaccines → prevent parasite development inside mosquitoes.
What is the life cycle of Plasmodium falciparum?
Mosquito injects sporozoites → enter liver.
Liver stage: sporozoites form merozoites.
Blood stage: merozoites infect RBCs → burst → symptoms (fever, anemia).
Some form gametocytes → taken up by another mosquito.
Inside mosquito → gametes fuse → zygote → ookinete → oocyst → sporozoites in salivary gland.
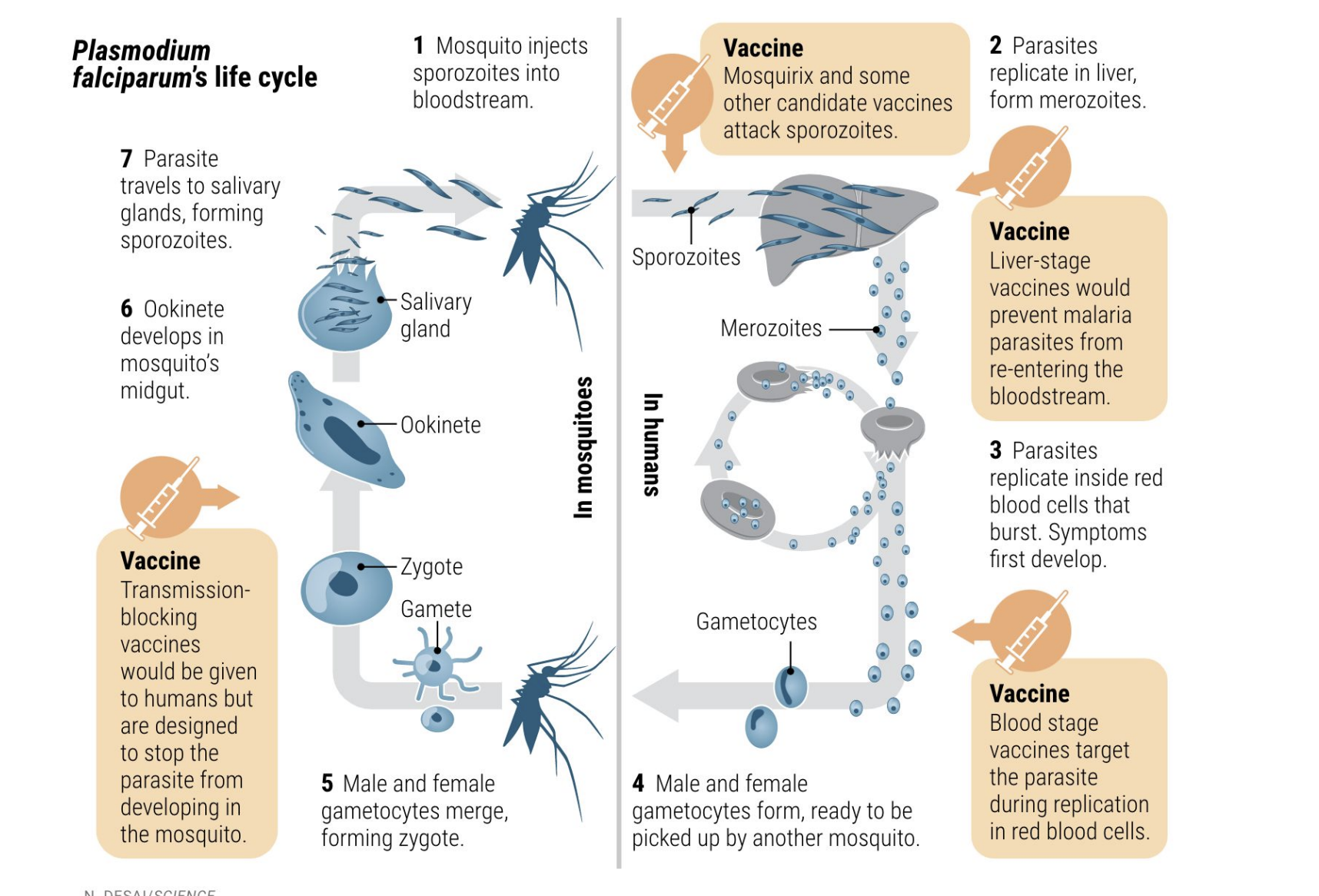
What is RTS,S?
First licensed malaria vaccine, developed in 1987, WHO-recommended.
Targets P. falciparum sporozoite (pre-erythrocytic) stage.
Given in 4 doses, provides ~30–50% immunity (wanes over time).
Reduces severe/deadly malaria by ~30%.
What are limitations or safety concerns of RTS,S?
Immunity wanes quickly.
Past trials noted possible ↑ risk of meningitis and female mortality, but not confirmed.
Despite this, WHO confirmed strong safety profile and cost-effectiveness.
What are the public health benefits of RTS,S?
Feasible to deliver and increases equitable access to malaria prevention.
Works synergistically with bed nets and other interventions.
Achieves >90% coverage when combined with existing tools.
How do mRNA vaccines work (general concept)?
mRNA encodes an antigen (e.g., spike protein) → body produces the protein → immune response.
Delivered in lipid nanoparticles (“fat thingies”) for protection + entry.
COVID-19 accelerated mRNA vaccine development from 8 years → ~1 year.
Future goal: broad, cross-reactive immunity (e.g., for seasonal or global pathogens).
Why is developing an HIV vaccine so difficult?
Rapid mutation → constant viral evolution and immune evasion.
Integrates into host genome → hides from immune system.
Targets CD4⁺ T cells, the very cells needed for immune response.
Attenuated vaccine would be too dangerous (risk of reverting to virulence).
What are broadly neutralizing antibodies (bNAbs)?
Special antibodies that neutralize multiple HIV-1 strains by binding conserved regions on the viral envelope.
Offer hope for a universal or long-lasting vaccine.
Some people naturally develop them after years of infection.
What are the challenges with bNAbs in vaccines?
Difficult to stimulate naturally in most people.
Some studies found that certain bNAbs increased HIV-1 acquisition instead of preventing it.
Ongoing research to engineer safe and effective bNAb-based vaccines.
What are the future directions in HIV vaccine research?
mRNA-based and personalized vaccine strategies under development.
Aim to mimic or induce bNAb-like responses.
A potential AIDS vaccine is in advanced trials (possibly within 2 years).
Researchers also exploring targeting different stages of the complex HIV life cycle.
When did agriculture first arise?
Around 12,000 years ago (early Holocene).
Plant domestication occurred independently in both the Old World (Afro-Eurasia) and New World (Americas).
However, animal domestication was limited mainly to the Near East (Fertile Crescent).
How did agriculture affect human health?
Transition to agriculture led to reduced dietary diversity, increased carbohydrate intake, and periodic food shortages → rise in obesity, Type II diabetes, and nutritional deficiencies.
What does the global domestication map show? (agriculture)
Early centers of plant domestication across Afro-Eurasia and the Americas (e.g., Fertile Crescent, Mesoamerica, East Asia, Andes).
Old World: multiple domestic animals → large-scale agriculture.
New World: fewer domesticable animals → smaller-scale agriculture.
How are humans an example of evolutionary mismatch?
MISMATCH: Negative consequence that results when a trait evolved in one environment is placed in another
Humans are Stone Age hunter-gatherers living in a modern industrial world.
• Genes adapted to scarcity + movement, but we now live in abundance + sedentariness.
• Leads to “diseases of civilization” — atherosclerosis, hypertension, diabetes, obesity.
What is a nutritional evolutionary mismatch?
→ Changes in diet due to agriculture + industrialization altered human nutrition:
↑ Glycemic load
Altered fatty acid ratio (↑ omega-6, ↓ omega-3)
Shifted macronutrient composition
↓ Micronutrient density
Altered acid–base balance
↑ Sodium–potassium ratio
↓ Fiber content
What is the impact of ultra-processed foods on health? (general ideas only)
→ Nutrient imbalance: high sugar, fat, salt; low fiber, micronutrients.
→ Rapid digestibility: quick glucose spikes → insulin resistance.
→ Additives/gut health: emulsifiers may harm microbiota.
→ Behavioral: hyper-palatable + convenient → overeating, higher calorie intake.
How do agriculture and industrialization link to chronic disease trends?
Diets became calorie-dense, less diverse → ↑ type II diabetes & obesity
→ Industrial revolution enabled large-scale farming → population boom + more processed foods.
→ Mismatch between ancient genes and modern lifestyle drives metabolic disease epidemic.
Why are “Paleo” diets misguided from an evolutionary perspective?
Evolution didn’t stop in the Pleistocene!
• Humans are omnivores — evolved to be dietarily flexible, not restricted.
• There was no single Paleolithic diet — it varied by geography and environment.
• Modern “Paleo” diets falsely assume humans reached a nutritional “pinnacle” then.
Evolution is ongoing — agriculture and later adaptations (like lactase persistence) show that.
What is lactase persistence and why is it important?
Ability to digest lactose in adulthood due to continued lactase enzyme activity.
• Most mammals lose lactase expression after weaning, but some humans retained it.
• This adaptation arose after dairy farming began — showing gene–culture coevolution.
→ Contradicts “Paleo” claim that our genome hasn’t changed since the Stone Age.
What is the Mediterranean diet’s effect on inflammation?
Promotes anti-inflammatory immune profile via:
• Healthy fats (olive oil, omega-3s) + antioxidants → ↓ chronic inflammation.
• B cells shift toward less inflammatory roles.
• Result: Balanced immune system → ↓ metabolic & chronic disease risk.
What is the CREBRF variant?
A “thrifty” allele found in some Polynesian populations (esp. Samoans).
• Promotes efficient energy storage as fat, allowing survival during periods of scarcity.
• Associated with higher BMI (≈ +1.5 per allele; +3 if homozygous).
What does “thrifty allele” mean?
A gene variant that enhances energy storage efficiency — advantageous in feast-famine cycles, but maladaptive in modern food-rich settings.
• Evolutionarily useful → survive starvation.
• Now → predisposes to obesity & type 2 diabetes in times of nutritional excess.
How does CREBRF function biologically?
Upregulated during starvation → helps preserve cells and maintain energy stores.
• In cell models:
↓ energy expenditure (↓ basal respiration, ↓ ATP production).
↑ lipid accumulation + triglycerides.
↓ cell death during starvation.
→ Overall: promotes “fuel efficiency” — good for survival, bad for caloric abundance.
What does CREBRF illustrate about gene–environment interaction?
Shows how genetic predisposition interacts with modern lifestyle.
• In a traditional subsistence setting, high energy storage = survival.
• In modern, calorie-rich environments, same variant = higher obesity & diabetes rates.
→ Great example of evolutionary mismatch in human health.
What are non-genetic contributors to high obesity/diabetes rates in Pacific populations?
Beyond genetics, colonialism and Westernization have played key roles:
• Rapid societal change + imported foods → loss of traditional diets.
• Structural factors: racism, socioeconomic inequality, and globalization.
✅ Takeaway: Obesity is not purely genetic — it’s a biocultural phenomenon.
What are allergy and autoimmune disorders?
Disorders where the immune system either overreacts to a trigger (e.g. pollen, peanuts, air pollution) or attacks self-tissues it shouldn’t (e.g. β-cells in pancreas → T1D; intestines → IBD).
Common features: Loss of immune tolerance, chronic immune activation, and interplay of genetic (polygenic) and environmental triggers.
Why is there a north–south gradient in multiple sclerosis (MS) and type 1 diabetes (T1D), and why does IBD correlate with wealth?
Higher rates in northern & wealthier countries.
Lower sunlight → ↓ vitamin D → weaker immune regulation.
Reduced pathogen exposure (“hygiene hypothesis”) — fewer infections early in life → undertrained immune system → higher risk of autoimmune & allergic diseases.
Reflects environmental + socioeconomic influences on immune-related disorders.
What is the original hygiene hypothesis?
Early childhood exposure to microorganisms helps train the immune system, protecting against allergic diseases by promoting immune tolerance.
What evidence supports the hygiene hypothesis?
Strachan (1989): Kids from large families had lower risk of hay fever and eczema → more early infection exposure.
Gerrard et al. (1976): Metis community had more helminth and infectious disease but less asthma/eczema than white community.
→ Suggests reduced pathogen exposure in cleaner environments increases allergy and autoimmune risk.
What is the refined hygiene hypothesis (aka old friends or biodiversity hypothesis)?
Early childhood exposure to co-evolved microorganisms protects against allergic and inflammatory diseases by promoting proper immune development.
What was the original mechanistic explanation of the hygiene hypothesis?
Infections (bacteria, protozoa) → activate Th1 immune cells.
Fewer infections → ↓ Th1 activity → ↑ Th2 activity → more allergic (Th2-mediated) disease.
Q: What evidence supports the hygiene hypothesis?
Rural < Urban allergy rates
Low-income < High-income countries
Early farm exposure
Helminth infections
Larger families (more sibling exposure)
What do the “old friends” and “biodiversity” hypotheses propose?
They suggest that the rise in allergies and inflammatory diseases is due to loss of co-evolved symbiotic microbes and parasites that once helped train and regulate the immune system.
→ Modern, industrialized lifestyles (clean environments, antibiotics, less farm/pet exposure) have reduced microbiome diversity.
How does loss of microbiome diversity relate to allergic disease?
The U.S. and other industrialized societies have far less microbiome diversity than hunter-gatherers.
Reduced microbial exposure in infancy disrupts immune development → ↑ risk of atopy (eczema, hay fever, asthma).
Protective early-life exposures: vaginal birth, breastfeeding, pets/farm animals, large families, infections (vs. antibiotics/formula).
What evidence links specific microbes to asthma protection? (Arrieta et al., 2015)
Infants at risk for asthma had ↓ levels of four bacterial genera (FLVR): Faecalibacterium, Lachnospira, Veillonella, Rothia.
Germ-free mice colonized with these bacteria had less airway inflammation, confirming a causal role for these taxa in preventing asthma.
Why are cesarean (C-section) births associated with higher rates of allergy and asthma?
C-section babies lack exposure to maternal vaginal microbiota during birth, leading to reduced gut microbiome diversity and altered immune development, which increases risk for allergic and asthmatic diseases.
Swabbing C-section infants with maternal vaginal fluids (“vaginal seeding”) can help transfer the mother’s microbiome, partially restoring microbial colonization similar to vaginally delivered infants.
What are Mendelian (single-gene) disorders?
Disorders caused by mutations in one gene.
Follow Mendelian inheritance (dominant or recessive)
Gene may be on an autosome or sex chromosome
Often easier to track in families
Examples: Cystic fibrosis, sickle-cell anemia, neurofibromatosis
What’s the difference between autosomal dominant, autosomal recessive, and X-linked inheritance?
Autosomal dominant: One mutant allele → disease (e.g., Marfan, Huntington’s, NF1)
Autosomal recessive: Two mutant alleles → disease (e.g., CF, Tay-Sachs, PKU)
X-linked: Mutation on X chromosome (e.g., Duchenne muscular dystrophy, hemophilia)
What is the relationship between allele frequency and effect size in genetic disorders? (Manolio et al., 2010)
Rare variants → large effect (Mendelian diseases)
Common variants → small effect (polygenic common diseases)
Low-frequency variants → intermediate effect
How do founder effects and population bottlenecks increase the burden of genetic disease?
When a small population becomes isolated, it carries only a subset of the original genetic variation.
→ Random genetic drift can make rare, deleterious alleles more common (founder effect).
→ Leads to higher rates of inherited disorders in small or isolated populations (e.g., Amish, Ashkenazi Jews).
Evolutionary processes affecting traits & disease:
Mutation – introduces new alleles
Genetic drift – random changes, strongest in small populations
Natural selection – favors beneficial traits
Gene flow – introduces variation through migration
Why are isolated populations valuable for studying genetic diseases?
Reduced genetic diversity due to founder effects and limited gene flow
High frequency of specific alleles, making it easier to map genotype–phenotype links
Stable environments & genealogical records (e.g., Amish, Ashkenazi Jews) simplify tracing inheritance
Enable identification of rare Mendelian mutations that are harder to find in diverse populations
How have founder effects shaped disease prevalence in isolated populations?
Amish & Mennonite communities: high rates of recessive disorders like Maple Syrup Urine Disease (MSUD) and MTHFR deficiency due to small founding populations and genetic drift
Ashkenazi Jewish population: descended from ~350 founders → higher frequency of disease alleles like Tay-Sachs, Gaucher, and Canavan
Both cases illustrate serial founder effect + cultural isolation, amplifying rare mutations
How has genetic counseling reduced Tay-Sachs disease prevalence in Ashkenazi Jews?
Autosomal recessive mutation in HEXA gene → buildup of GM₂ ganglioside → neuronal death, “cherry-red spot” on retina
Carrier screening (Dor Yeshorim program):
Confidential genetic testing & couple compatibility checks before marriage
Greatly reduced Tay-Sachs frequency to rates similar to non-Jewish populations
Also seen in Cajun and Quebecois populations
What evidence suggests a connection between Tay-Sachs disease and tuberculosis resistance?
HEXA mutation frequency in Ashkenazi Jews correlated with regions of historically high TB prevalence.
For Eastern European–born grandparents of Ashkenazi children with Tay-Sachs, only 1 of 306 had died from TB, despite TB causing up to 20% of deaths in Eastern European cities in the early 1900s.
Suggests a historical selection pressure where TB may have influenced the persistence of HEXA mutations.
How might Tay-Sachs carriers have increased resistance to tuberculosis?
Carriers produce more β-subunit of hexosaminidase (Fernandes Filho & Shapiro, 2004).
The β-subunit enhances host defense against Mycobacterium tuberculosis (Koo et al., 2008).
This could represent a form of heterozygote advantage, where carrying one HEXA mutation provides protection against TB without causing disease.
What are the main methods for detecting recent human adaptation?
Change in Site Frequency Spectrum (SFS) — detects deviations from neutral allele frequency.
Change in F<sub>ST</sub> — finds regions of high differentiation across populations.
Locus-Specific Branch Length (LSBL)/PBS — measures population-specific divergence.
Extended Haplotype Homozygosity (EHH) — identifies long haplotypes (selective sweeps).
GWAS — finds alleles associated with adaptive traits.
What are the main types of natural selection and their effects?
Purifying selection: Removes harmful alleles → rare, severe diseases.
Positive selection: Favors advantageous alleles → rapid allele rise, common traits.
Balancing selection: Maintains multiple alleles → heterozygote advantage (e.g., sickle cell).
How did lactase persistence evolve and why is it an example of convergent evolution?
Mutation near MCM6 gene → continued expression of lactase enzyme into adulthood.
Independent mutations arose in Europeans and Africans (~8,000 years ago) with cattle domestication.
Autosomal dominant trait — evolved due to strong selection for milk digestion (calcium, hydration).
Convergent evolution: Same phenotype evolved separately in distinct populations.
What does a long haplotype indicate in lactase persistence genes?
Long haplotypes = recent, rapid selection → “selective sweep.”
Suggests lactase persistence spread quickly due to a strong adaptive advantage.
How have humans adapted to high-altitude hypoxia?
Lower oxygen → selection for efficient oxygen use via HIF pathway genes (e.g., EGLN1, EPAS1).
Tibetans: Carry EPAS1 variant introgressed from Denisovans; lower Hb levels but higher O₂ efficiency.
Andeans: Increased Hb concentration and nitric oxide for vasodilation.
Ethiopians: Distinct genetic mechanisms yet similar adaptation — convergent evolution.
What is convergent evolution and give an example?
Definition: When unrelated species/populations independently evolve similar traits.
Examples:
Sugar gliders (marsupial) & flying squirrels (placental mammals).
African & European lactase persistence mutations.
Tibetan and Andean altitude adaptations.