Bioavailability
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Bioavailability
• The fraction of an administered dose of unchanged drug that reaches the systemic circulation.
• Rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action.
less than or equal to 1
• A number__________ that indicates the fraction of drug reaching the systemic circulation.
Bioavailability formula
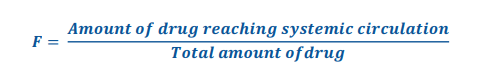
Absolute Bioavailability
• Compares the bioavailability of the active drug in the systemic circulation following extravascular administration with the bioavailability of the same drug following intravenous administration
= close to 1 (0.99 / 100%) = efficient good
Absolute Bioavailability formuala
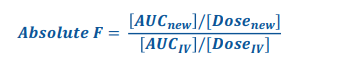
Relative Bioavailability
• The systemic exposure of a drug in a designated formulation (generally referred to as treatment A or reference formulation) is compared with that of the same drug administered in a reference formulation (generally referred to as treatment B or test formulation).
Relative Bioavailability formula
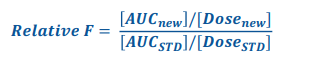
Frel >1 =
High systemic delivery cmprd to std
Frel <1 =
Low systemic delivery cmprd to std
• Physicochemical properties
• Gastric emptying rate
• Metabolism in intestinal wall
• Drug efflux from intestinal wall
• Hepatic first pass metabolism
Factors that Affect Bioavailability