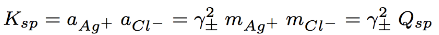Basic Thermodynamics II
1/29
Earn XP
Description and Tags
Exp. 2 - Silver Equilibrium
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
ideal dilute solutions
Solutions for which the solute obeys Henry’s law and the solvent obeys Raoult’s law
Henry’s law
pB = XBKB
in very dilute solutions, the vapor pressure of the solute is proportional to its mole fraction
Henry’s Law solute chemical potential
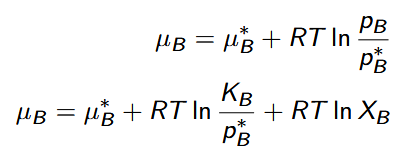
chemical potential of a solute in an ideal dilute solution
μ† = μ∗B + RT ln KB /p∗B
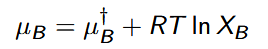
activity, ai
accounts for deviations from ideal behavior of solutions
aA = pA / p∗A
activity coefficient, γi
quantifies the degree to which the solution is non-ideal, or it measures the extent to which the effective concentration is different from the true concentration, Xi; depends on the composition, molality, and temperature of the solution
ai = γiXi
μ = μ◦ + RT ln a
a = γm
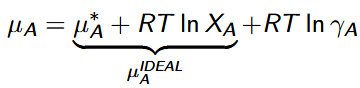
activity coeffecient for solvent
γA → 1 as XA → 1 at all temperatures and pressures.
activity coeffecient for solute
γB → 1 as XB → 0 at all temperatures and pressures.
Electrolytes
exist as solvated positive and negative ions; quite different from the ideal and real (nonelectrolyte) solutions of neutral solutes
electron interactions
Coulomb interactions between ions in an electrolyte solution are so strong that approximation of replacing activities by molalities is valid only in very dilute solutions (< 10−3 mol kg−1 in total ion concentration)
chemical potential of electrolyte solute
Mp Xq (s) H2O → pMz+ (aq) + qX z− (aq)
pz+ + qz− = 0
s = p + q
μB = μ◦B + RT ln aB
μB = pμ+ + qμ−
a+ = γ+m+ and a− = γ−m−
mean ionic chemical potential μ±
μ± = μB = pμ+ + qμ−
mean ionic molality m±
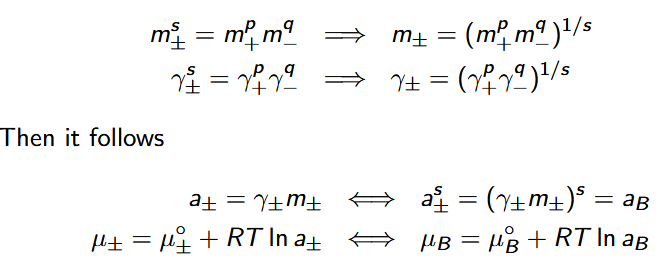
Debye-Hückel limiting law
og γ± = −|z+z−|AI ½
where A = 0.5092 at T = 25◦C and I is the ionic strength of the
solution defined by
I = ½ ∑i z2i mi
In this expression zi is the charge of an ion i and mi is its molality
molality and molarity
in case of very dilute aqueous solutions, a volume of 1 L has a mass of 1 kg, so ___ have nearly the same values
electrochemical cell
consists of two electrodes (metallic conductors), in contact with electrolyte (an ionic conductor); they form two half-cells (compartments); difference in the electrical potential (voltage) between the electrode and the solution in each of the two half-cells;
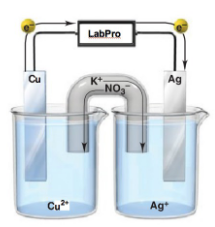
salt bridge
when electrolytes different, the two compartments are joined to complete the electrical circuit and enable the cells to function
galvanic (Daniell) cell
type of electrochemical cell that produces electricity as a result of the spontaneous chemical reaction occurring inside it
reaction quotient Q
Q = activities of products
activities of reactants = ∏j ajνj
extent of reaction ξ
dimension of amount of substance and is reported in moles
Gibbs Free Energy given change in extent of reaction
∆G = = ∆G ◦ + RT ln Q
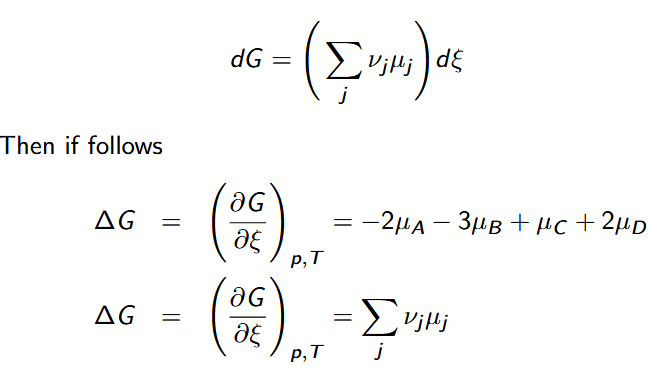
Nernst equation
∆G = −νFEcell
Ecell = E◦cell − (RT/νF) ln Q
use a to account for non-ideality
Faraday’s constant
the charge per mole of electrons = −eNA = 9.6485×104 C/mol
redox reactions
type of chemical reaction in which the oxidation states of the reactants change; one loses electrons (oxidation), one gains electrons (reduction)
Universal Electrode Vessel
our version of the electrochemical cell
Cu electrode
CuSO4(aq)
Ag electrode
Salt bridge - KNO3(aq) + agar gel
Fritted glass or small hole
Magnetic stirring bar
Thermometer
Stirring plate

Reaction mixture start
1.00 x 10-4 M AgNO3(aq)
1.00 x 10-3 M NH4NO3(aq)
work-horse equation
1) Obtain the apparent value of the “const.” from an initial measurement of Ecell with a solution of known mAg+ ≈ [Ag+]. (calibration step)
2) Then use “const.” and Ecell to deduce mAg+ ≈ [Ag+] when other equilibria are taking place (i.e. when you add other reagents)
3) Follow the lab write-up for calculations.
![<p>1) Obtain the apparent value of the “const.” from an initial measurement of Ecell with a solution of known mAg+ ≈ [Ag+]. (calibration step)</p><p>2) Then use “const.” and Ecell to deduce mAg+ ≈ [Ag+] when other equilibria are taking place (i.e. when you add other reagents)</p><p>3) Follow the lab write-up for calculations.</p>](https://knowt-user-attachments.s3.amazonaws.com/006e58f7-b573-48a2-bfc1-afbaea567630.png)
precipitate forms
Since the initial values of silver and chloride concentration were equal before precipitation (equal number of moles were added), after the precipitate forms the concentrations remaining in solution will also be equal
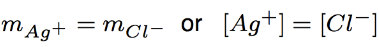
ion product
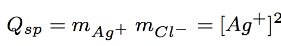
solubility product