Rate Equations
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
The rate of a reaction is the increase in the amount of ………created in the reaction over………
product
time
There are ways we measure the change in the amount of substance for example: for change in the amount of solid is measured by the …….. and in gases its the ……….
BUT the change in ………… is what we use to accurately measure the change in a ……….
mass
volume
concentration
solution
We define concentrion in liquids as: the number of particles of ……. in a particular ……… of solution
The concnentration of gas : the number of particles of ….. in a particular …………
solute
volume
gas
volume
to find rate of reaction: change in ……………/ hange in ……….
so the units for rate of reaction are :
concentration
time
moldm^-3s^-1
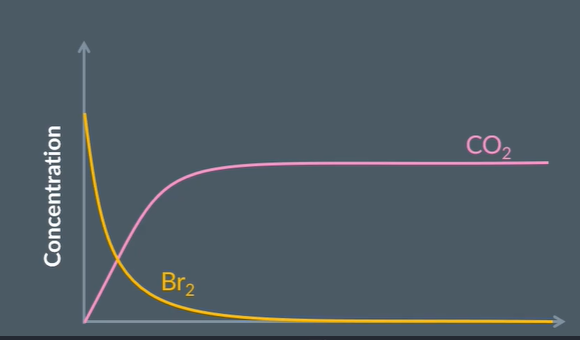
We can measure the rate of reaction by measuring the change of concnetration of reactants
The pink line shows the ………. of CO2(..) over time wheras the orange line shows the ………. of Br2 (..) over time as the reaction progresses
both curves are steep at first as the recation is ………. occuring but start to level off which means teh reaction is ……… down
concnetration
g
concnetraion
aq
fast
slowing
Why does the reaction slow down?
As the concentration of carbon dioxide increases, it prevents the reaction from progressing further.
As the concentration of reactants decreases, the proportion of particles with sufficient activation energy decreases.
As the concentration of reactants decreases, the frequency of collisions decreases.
As the concentration of products increases, the activation energy decreases.
C (untill all teh reactants have been used up and the recation has finished)
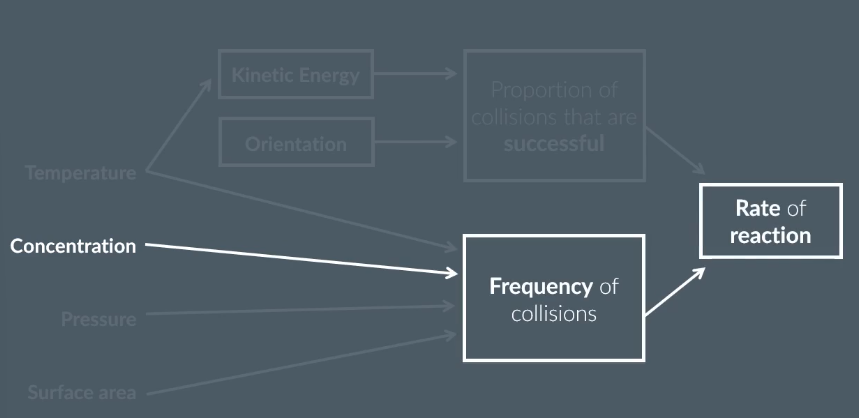
The rate of reaction is…
Select all that apply
measured in mol dm−3s1
the change in the amount of reactant or product over time
the amount of heat given out by a reaction over time
the change in concentration of products
B
(X A : meaures in moldm-3s-1)
(X D : not products oly reactants)
the rate of reaction can also be defined as:
The amount of one ……… used up over time
The amout of one ………. ceated over time
reactant
product
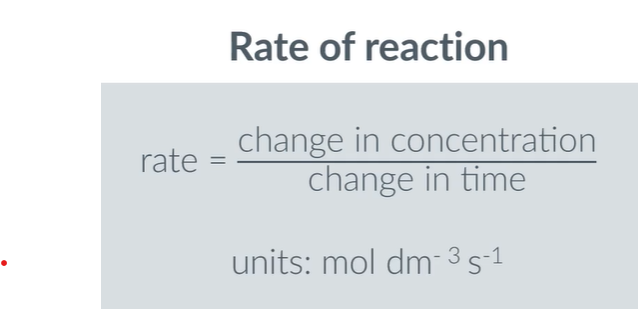
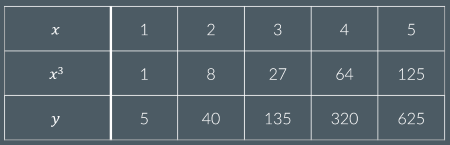
If y∝x3, what is k?
K=5
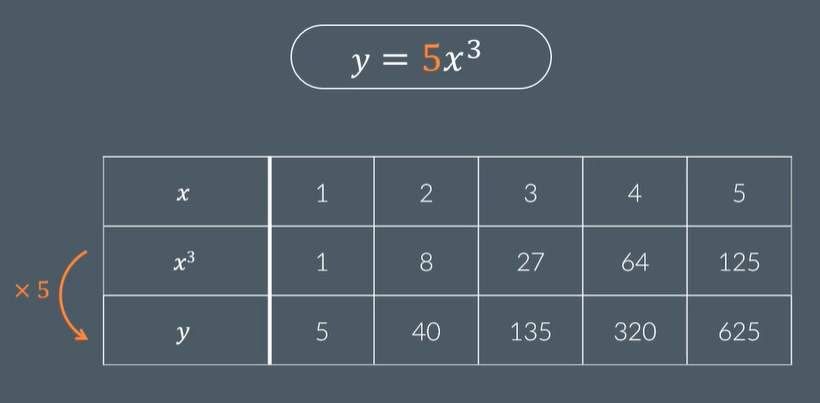
y=kx^7 means that y is ……. to x to the power of 7.
proportional
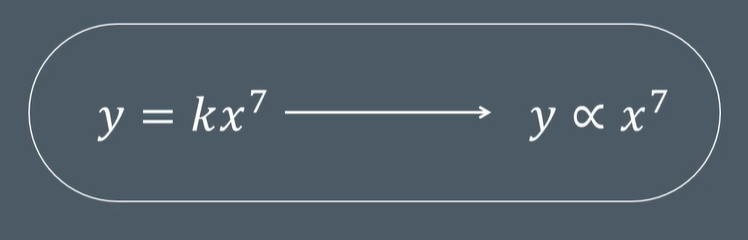
A proportionality constant is used to…
work out the value of 𝑥 x .
work out which power to use.
identify a proportional relationship.
quantify a proportional relationship.
D

Look at the table below. Are 𝑥 x and 𝑦 y proportional?
Yes : If x increases by a given factor, y increases by the same factor. For example, when x increases by a factor of 2, y also increases by a factor of 2.
Look at the table below. Are x2 and y proportional?

No: When x2 increases by a given factor, y increases by a different factor. This means that x2 and y are not proportional to each other.
Look at the table below. Are x and y proportional?
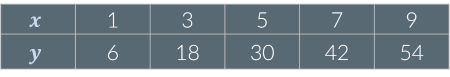
Yes: If x increases by a given factor, y increases by the same factor. For example, when x increases by a factor of 3, y also increases by a factor of 3.
Look at the table below. Are x2 and y proportional?
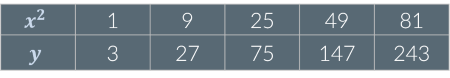
yes: If x2 increases by a given factor, y increases by the same factor. For example, when x2 increases by a factor of 9, y also increases by a factor of 9.
Look at the table below. Are x2 and y proportional?

No: When 𝑥² increases by a given factor, 𝑦 increases by a different factor. This means that 𝑥² and 𝑦 are not proportional to each other.
Look at the table below. Are x2 and y proportional?

Yes: If x² increases by a given factor, y increases by the same factor. For example, when x² increases by a factor of 4, y also increases by a factor of 4.
Look at the table below. Are x and y proportional?
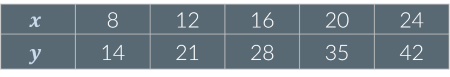
No: If x increases by a given factor, y increases by the same factor. For example, when x increases by a factor of 1.5, y also increases by a factor of 1.5.
Look at the table below. Are x2 and y proportional?

No: When x² increases by a given factor, y increases by a different factor. This means that x² and y are not proportional to each other.
Given the following data, y is…
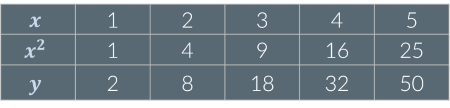
Not proportional to x or x²
Proportional to x
Proportional to x²
Proportional to x²: If x² increases by a given factor, y increases by the same factor. For example, when x² increases by a factor of 4, y also increases by a factor of 4.
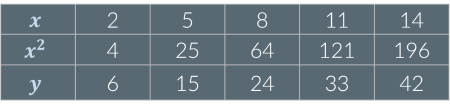
Given the following data, y is…

Not proportional to x or x2
Proportional to x
Proportional to x2
A :When x or x² increase by a given factor, y increases by a different factor. This means that neither x nor x² are proportional to y.
Given the following data, y is…
Not proportional to x or x2
Proportional to x
Proportional to x2
B; If x increases by a given factor, y increases by the same factor. For example, when x increases by a factor of 7, y also increases by a factor of 7.
Given the following data, y is…
Not proportional to x or x2
Proportional to x
Proportional to x2
B: If x decreases by a given factor, y decreases by the same factor. For example, when x decreases by a factor of 2, y also decreases by a factor of 2.
Given the table of data, y is…
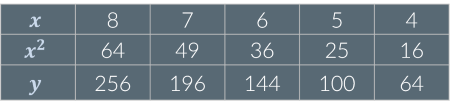
Not proportional to x or x2
Proportional to x
Proportional to x2
C: If x² decreases by a given factor, y decreases by the same factor. For example, when x² decreases by a factor of 4, y also decreases by a factor of 4.
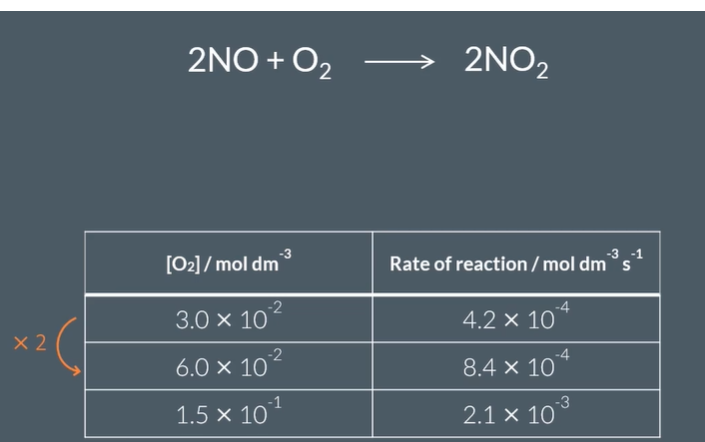
When Caleys initial reactanta concnentration increase by 2 to produce 2 times the rate, The rate of Caleys reaction is ………… to the …………. of oxygen
proportional
concentration
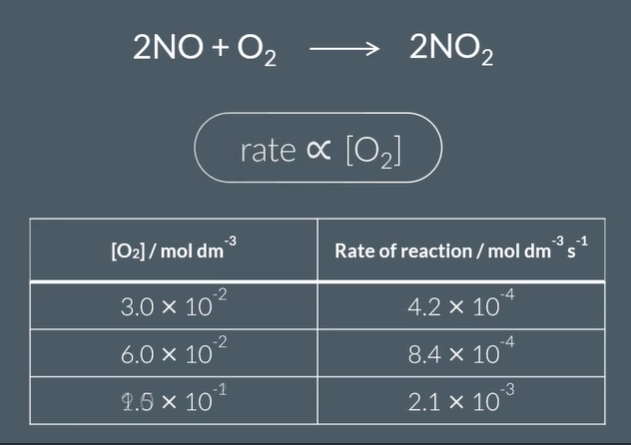
The rate of reaction is proportional to...

[ O_2] ^…….
[O2]²
![<p>[O2]²</p>](https://knowt-user-attachments.s3.amazonaws.com/7261e6b1-5179-4150-8839-933e5dcb1b22.png)
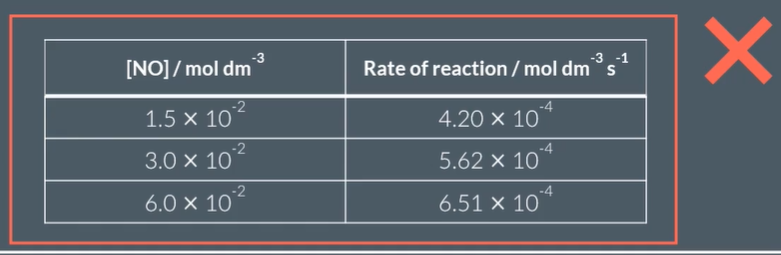
Is this a proportional relationship?
yes: rate is proportional to [NO]
yes: rate is proportional to [NO]²
no
C: by no factors when dividing exp 1 con and rate values for any other
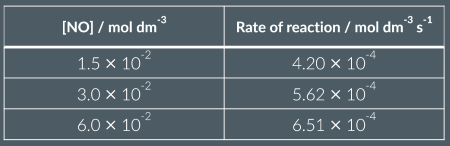
Is this a proportional relationship?
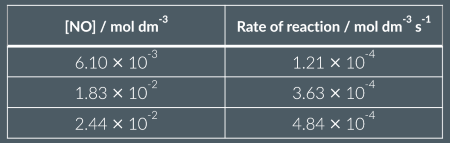
yes: rate is proportional to [NO]
yes: rate is proportional to [NO]²
no
A: when dividing conc and reacs of exp 1 by any other to get a factor of 3 ( as conc triples , rate of reaction triples) as also when the ror quadruples the conce of exp 3 in rel to exp 1 quadruples)
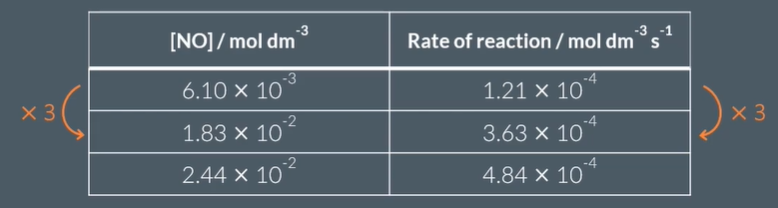
We can tell that there’s a …………relationship between the rate of reaction and the concentration of a………..by looking for mathematical relationships in the data from an………..
proportional
reactant
experiment
6 orders of reaction