Gas Transport: CO2 Transport
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Steady-State Arteriovenous CO2 Blood Gas values
PvCO2 = 45 mmHg
PaCO2 = 40 mmHg
Arterial [CO2] = 48 vol%
Mixed Venous [CO2] = 52 vol%
maintained blood levels: 48-52 vol%
excess eliminated: 4-5 vol%
![<p>PvCO2 = 45 mmHg</p><p>PaCO2 = 40 mmHg</p><p>Arterial [CO2] = 48 vol%</p><p>Mixed Venous [CO2] = 52 vol%</p><p>maintained blood levels: 48-52 vol%</p><p>excess eliminated: 4-5 vol%</p>](https://knowt-user-attachments.s3.amazonaws.com/70fcbad1-d653-48e0-91bc-6dea9eaf1990.png)
CO2 movement into the blood
Venous [CO2] – Arterial [CO2]
52 - 48 = 4 vol% at rest
- CO2 produced from oxidative metabolism and exits tissue capillaries via diffusion across the blood tissue barrier
- about the same amount of O2 taken from the blood by tissues to fuel oxidative metabolism
total volume of CO2 in blood
~3L CO2 in 6L blood volume
- 3x more than O2
blood CO2 as a major determinant of pH
- hydration of CO2 results in the formation of H+
- H+ level relative to HCO3- determines pH
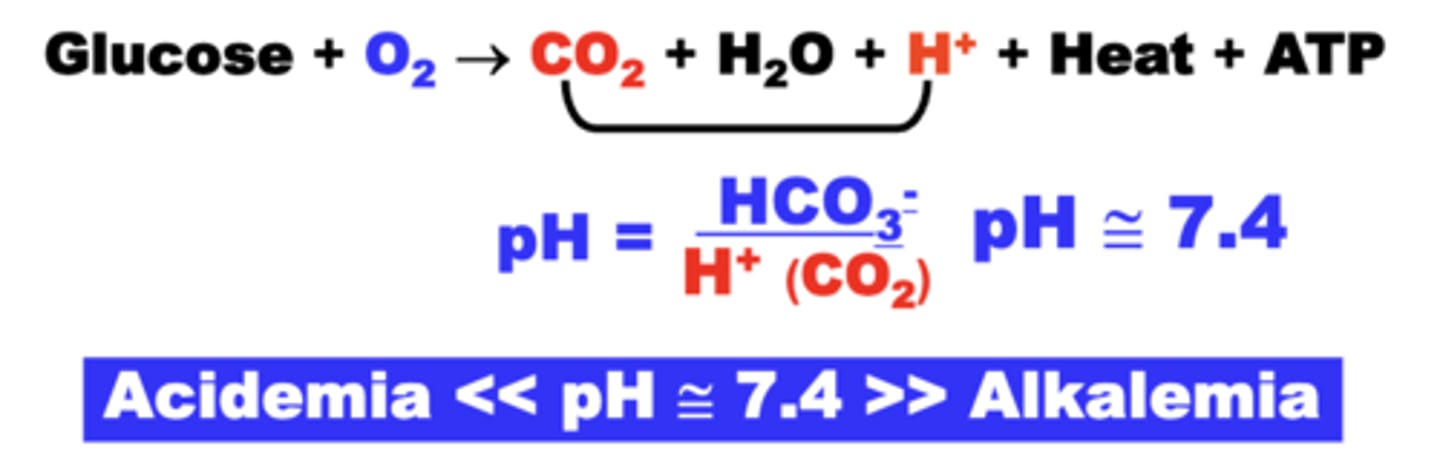
normal physiological pH
pH maintained around 7.4
> 7.4 = Alkalemia (decreased PCO2)
< 7.4 = Acidemia (increased PCO2)
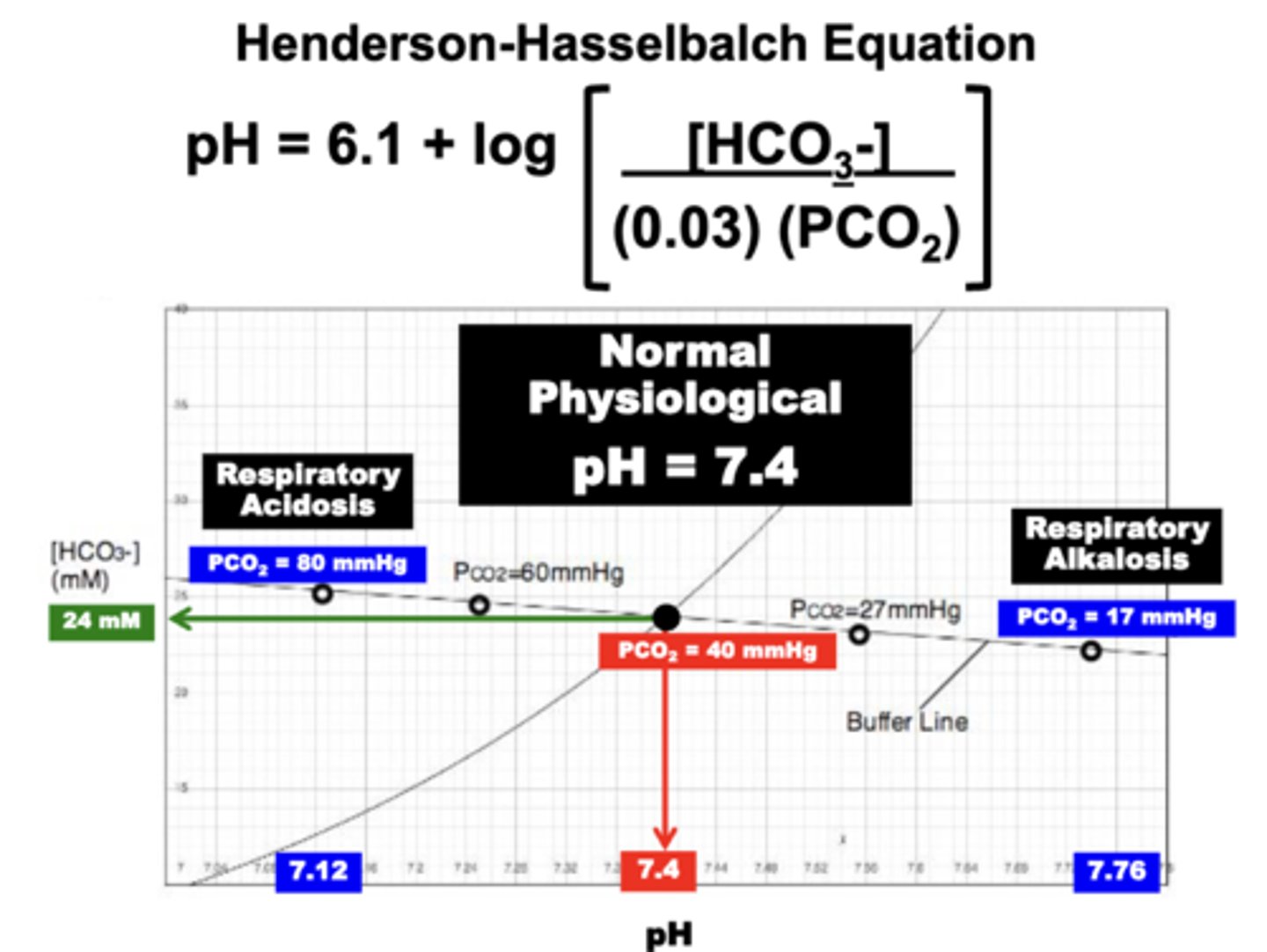
henderson-hasselbach equation
relates the principle variables determining pH
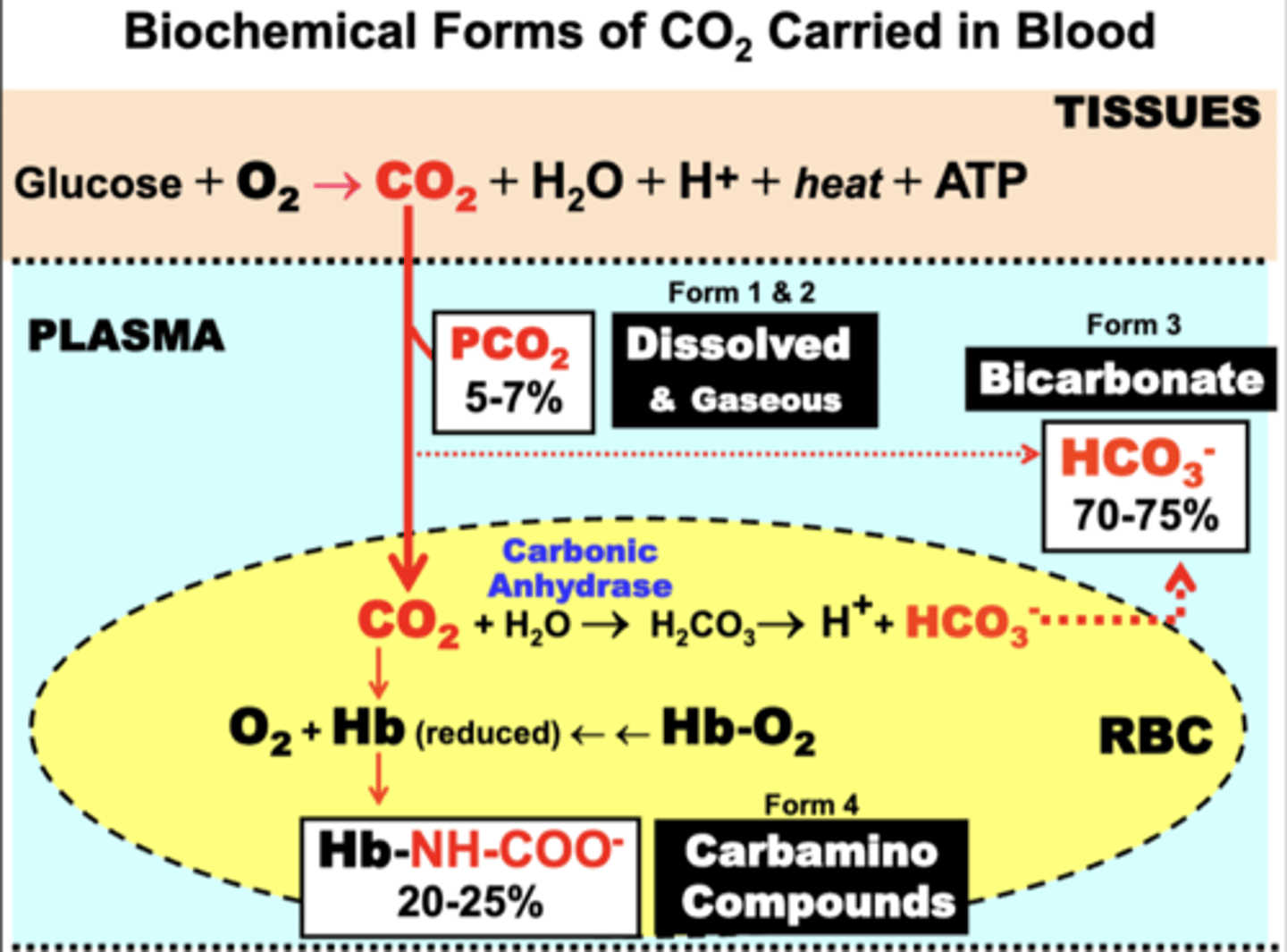
Blood CO2 is carried as what biochemical forms within which compartments?
compartments:
- Tissue
- Plasma
- RBC
forms:
1. gaseous (plasma)
2. dissolved (plasma)
3. bicarbonate
4. carbamino compounds
- carbaminohemoglobin
gaseous + dissolved CO2
dissolved:
- 5-7% of total CO2 in plasma
- obeys Henry's Law
- is proportional to the plasma PCO2 and inherent CO2 solubility in plasma
= arterial [CO2] = 2.4 vol%
= mixed venous [CO2] = 2.7 vol%
gaseous:
- minute amounts
both are measured as the PCO2
CO2 vs O2 solubility in plasma
CO2 solubility in plasma is 20x greater than O2
- 0.06 ml/100ml/mm Hg (CO2)
- 0.003 ml/100 ml blood/mm Hg (O2)
Due to the limited volume of dissolved CO2, greater blood CO2 transport is accomplished by?
biochemically converting CO2 to more soluble forms
bicarbonate
70-75% (35-38 vol%) of total CO2 in blood is carried as Bicarbonate (HCO3-)
HCO3- is formed from dissolved CO2 through the reversible biochemical reaction:
CO2 + H2O <--> H2CO3 <--> HCO3- + H+
Carbamino Compounds
20-25% (10-12 vol%) of total CO2 is carried as Carbamino Compounds
Carbamino compounds are formed by the reversible combination of CO2 with the terminal amine group of blood proteins
Carbaminohemoglobin
The most important is the globin moiety of Hb
Hb-NH2 + CO2 <--> Hb-NH-COO- (carbaminohemoglobin) + H+
HbO2 unloading
PO2-dependent
process:
- fully oxygenated Hb (Hb-O2) is transported by RBCs in arterial blood (plasma)
- Hb O2 unloading is initiated by the diffusion of dissolved O2 down the PO2 gradient from systemic capillaries (PaO2) into tissues (PTO2)
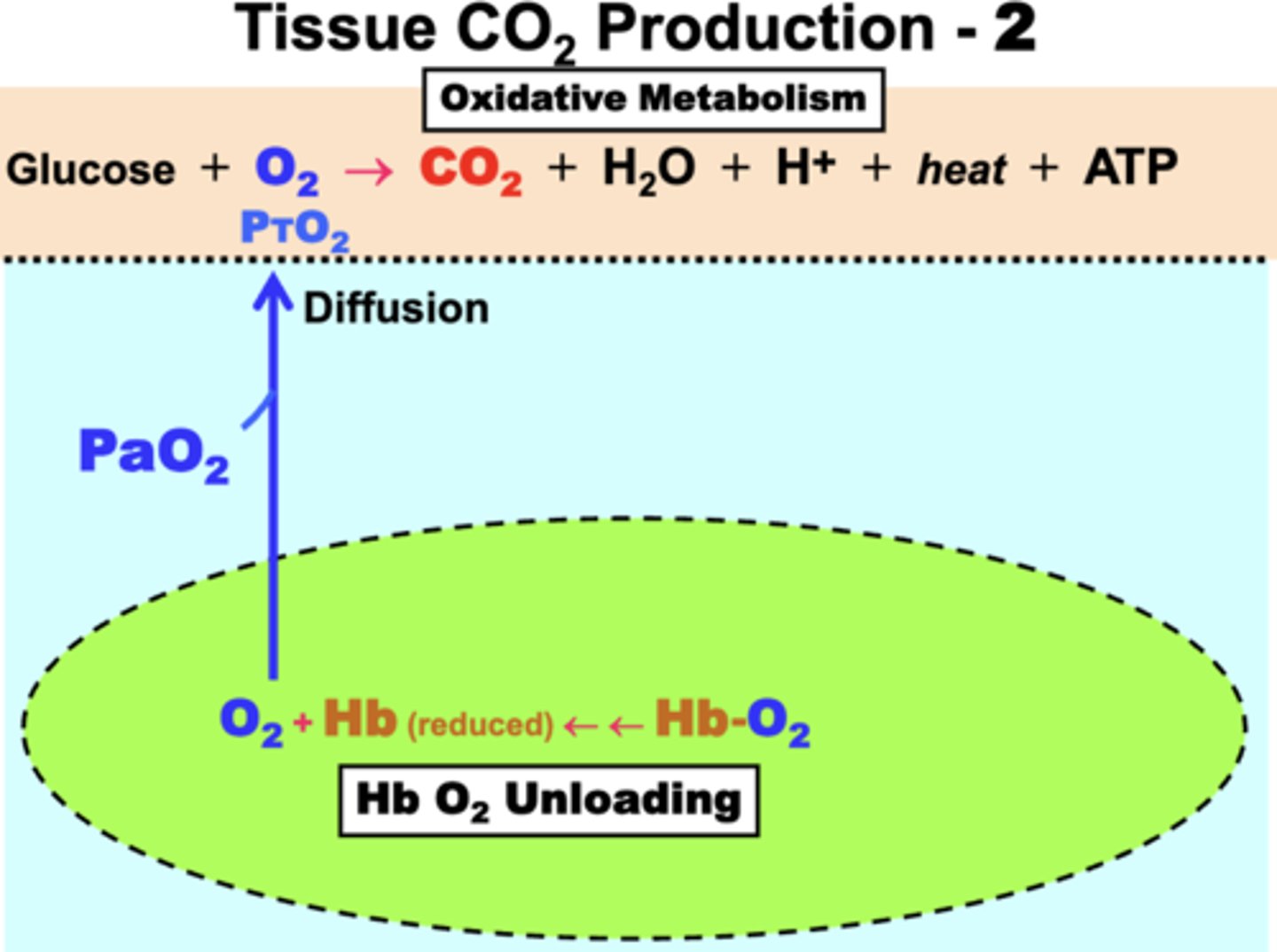
tissue O2 consumption produces?
CO2, H+, & heat as waste products of the oxidative metabolism
- CO2 is eliminated by movement into blood
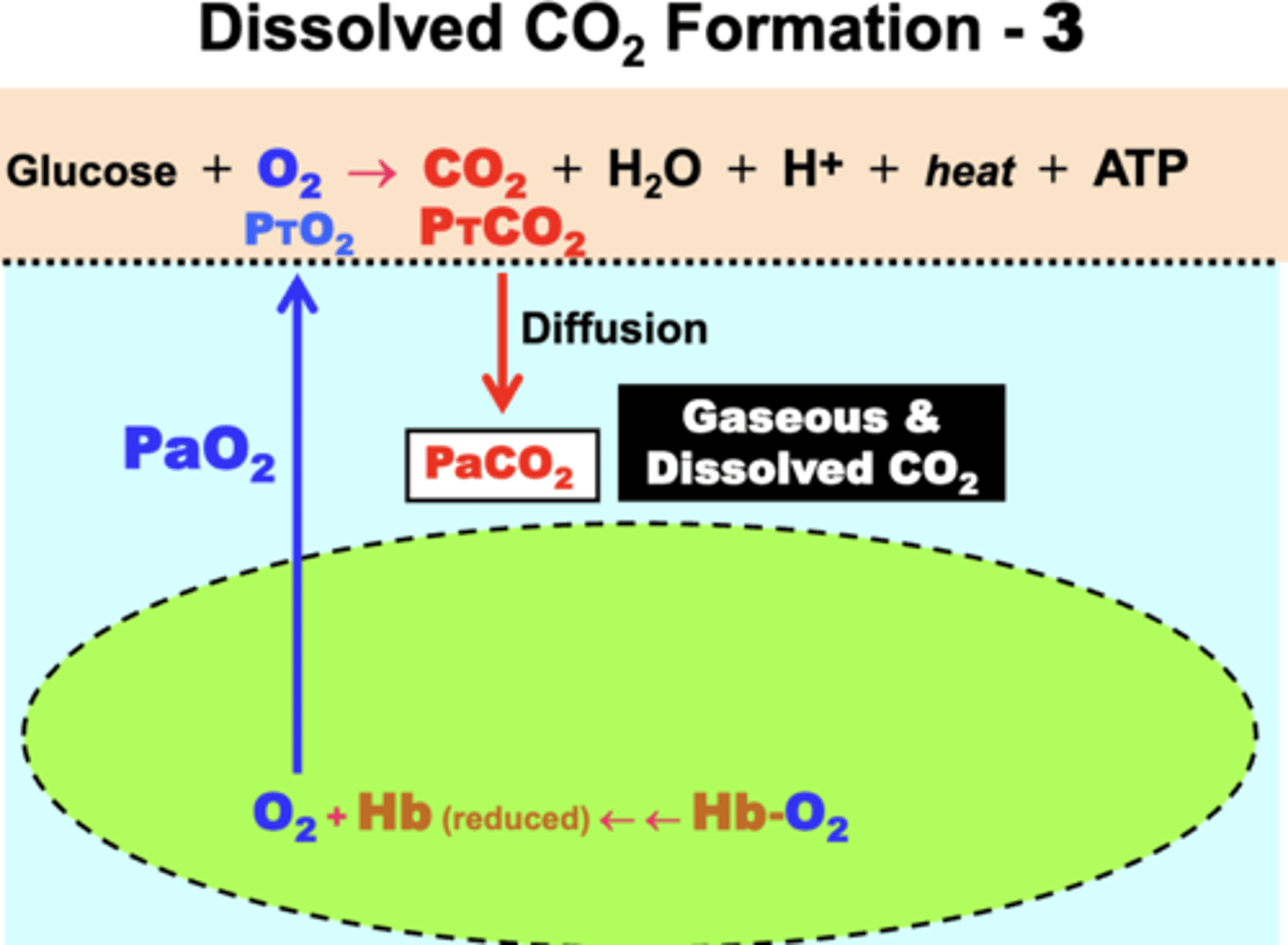
dissolved CO2 formation
1. CO2 diffuses down its PCO2 gradient from tissue (PTCO2 ) into plasma (PaCO2)
2. some is solubilized into dissolved CO2 as a result of the elevated gaseous pressure (PaCO2)
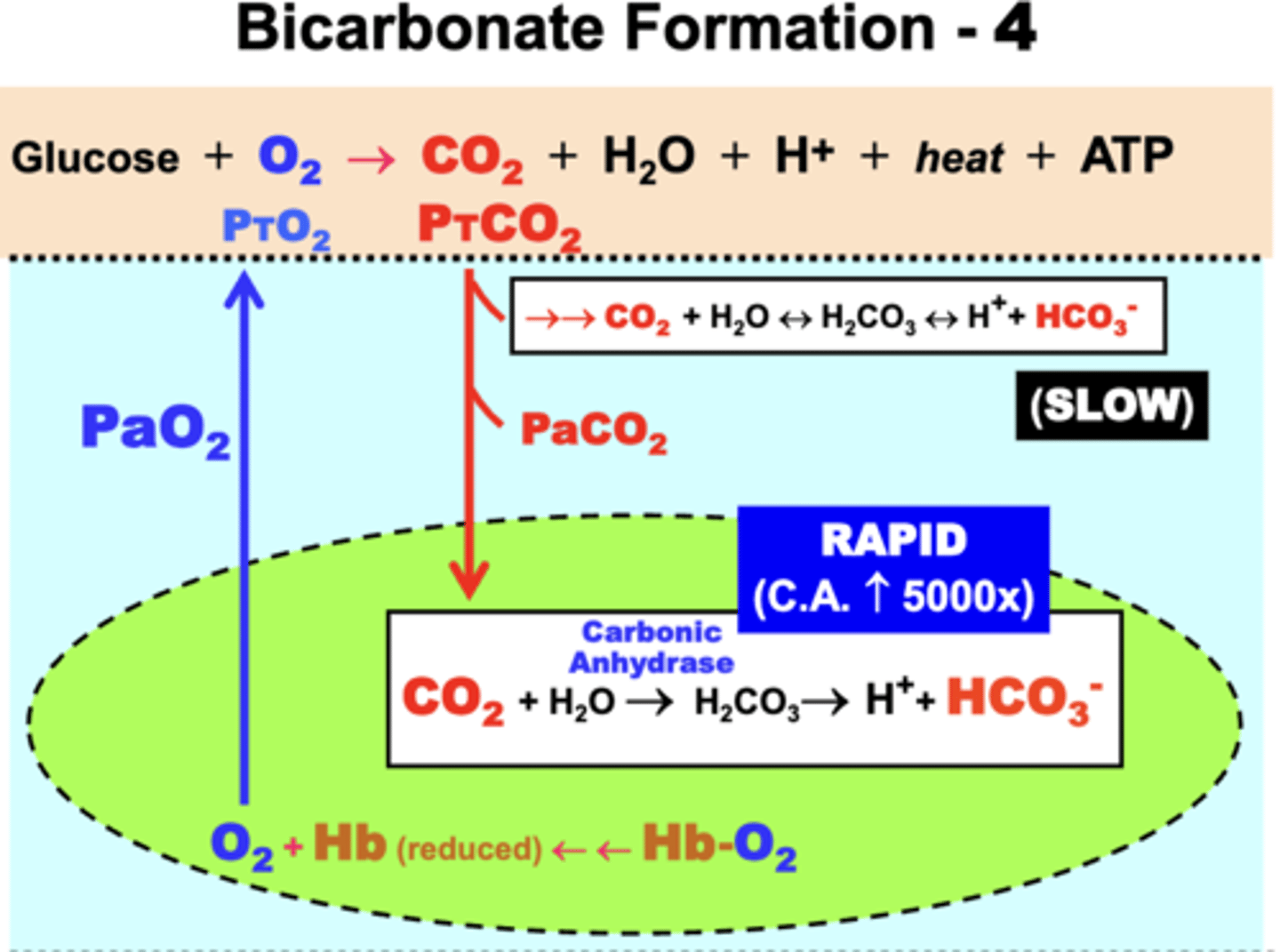
bicarbonate formation
in plasma:
- some CO2 becomes hydrated in plasma in an equilibrium reaction
- forms the important acid-base products bicarbonate (HCO3-) and hydrogen ions (H+)
- rate of this reaction in plasma is slow
- slow reaction causes most CO2 to diffuse into RBCs
in RBCs:
- CO2 conversion to HCO3- and H+ is very rapid (5000x greater) due to presence of the catalytic enzyme Carbonic Anhydrase (CA)
electrochemical gradient between RBCs and plasma
Because a disproportionate amount of CO2 conversion occurs within RBCs ->
- 12-fold [H+] & [HCO3-] Gradients develop between RBCs and the plasma
- HCO3- readily diffuses out of RBCs into plasma
Electrical Gradient:
- cations such as H+ do NOT easily cross cell membranes
- causes more net anion (HCO3-) flux into plasma
- forms a net electrical gradients across RBC membranes (inside more + relative to the outside)
![<p>Because a disproportionate amount of CO2 conversion occurs within RBCs -></p><p>- 12-fold [H+] & [HCO3-] Gradients develop between RBCs and the plasma</p><p>- HCO3- readily diffuses out of RBCs into plasma</p><p>Electrical Gradient:</p><p>- cations such as H+ do NOT easily cross cell membranes</p><p>- causes more net anion (HCO3-) flux into plasma</p><p>- forms a net electrical gradients across RBC membranes (inside more + relative to the outside)</p>](https://knowt-user-attachments.s3.amazonaws.com/32ce47f7-2095-4197-a2e5-5a9f87f5504c.png)
electrochemical gradients and accumulation of reduced Hb in RBCs account for?
the biochemical mechanisms related to the Bohr Effect
Bohr Effect Mechanism
As Hb is progressively desaturated → availability of deoxy-Hb results in formation of carbamino-Hb
two effects:
1. Carbamino-Hb formation maintains the PCO2 gradient between tissues and blood:
- allows more CO2 to diffuse into blood for the same PCO2 difference in proportion to Hb %SO2
2. Carbamino-Hb formation prevents rebinding of O2 to deoxy-Hb:
- reduces Hb-O2 affinity and favors greater O2 diffusion into tissues as more CO2 moves into blood (Bohr Effect)
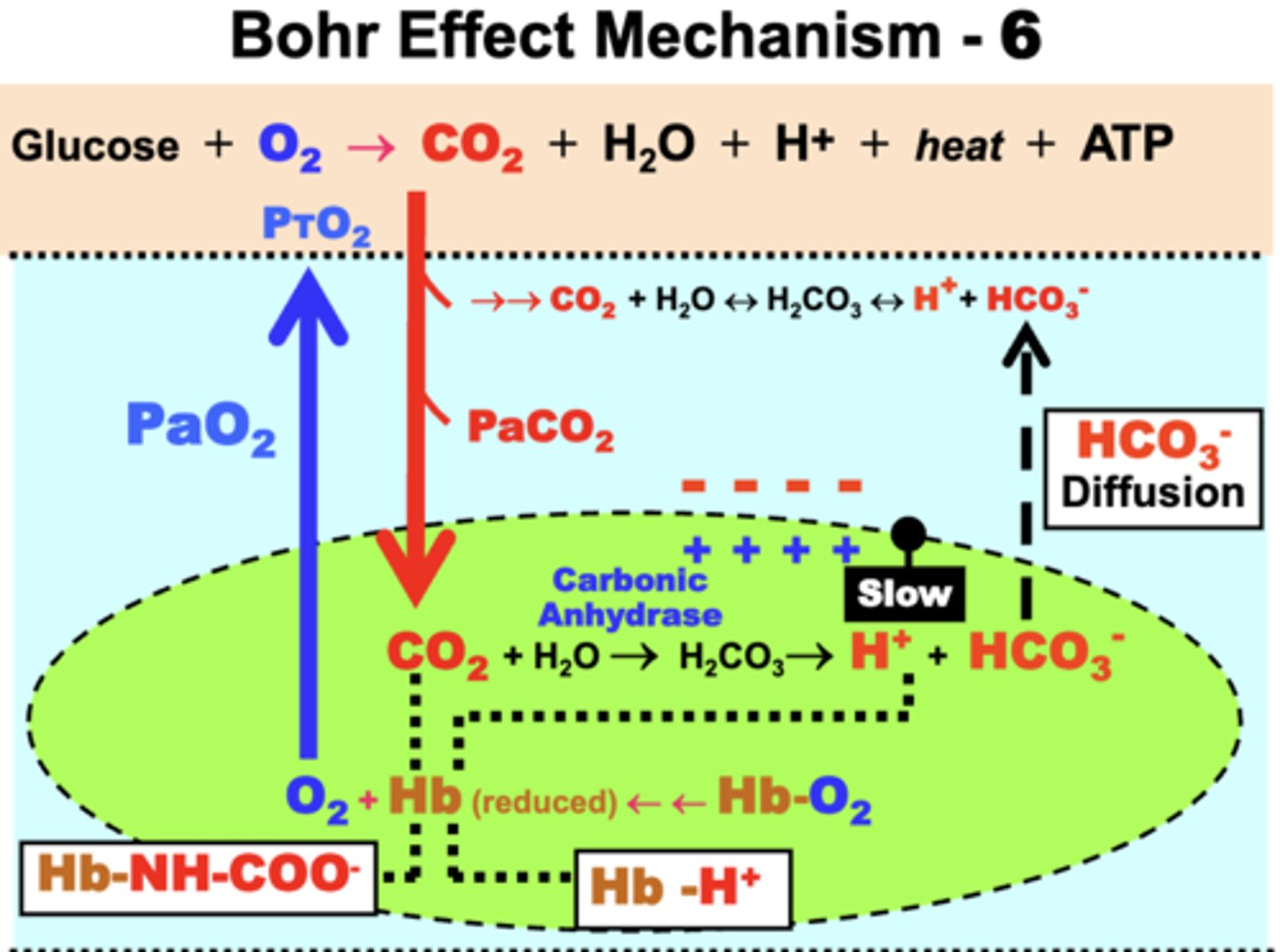
reduced Hb's effects on Bohr Effect
- deoxy-Hb
- a strong proton acceptor
- forms acid Hb by reacting with accumulated H+ in RBCs
two effects:
1. Free [H+] is kept low in RBCs:
- favors CO2 hydration by mass action ([HCO3-] is low because it easily diffuses out)
- more CO2 can then move into blood as Hb %SO2 decreases (i.e. increased CO2 levels)
▪ Hb-H+ formation also keeps [H+] low in plasma → acts as an important blood buffering mechanism
2. Hb-H+ formation prevents O2 rebinding by Hb:
- favors greater O2 diffusion into tissues as CO2 blood transport increases (Bohr Effect)
Chloride Shift
mechanism that functions to maintain or restore cell homeostasis
- to correct the electrical gradient resulting from net cation (HCO3-) efflux from RBCs, chloride ions (Cl-) diffuse from the plasma into RBCs to restore cell electroneutrality
consequence: the total ion concentration in RBCs is now greater than in plasma venous blood
- creates an osmotic gradient that causes H2O to enter RBCs
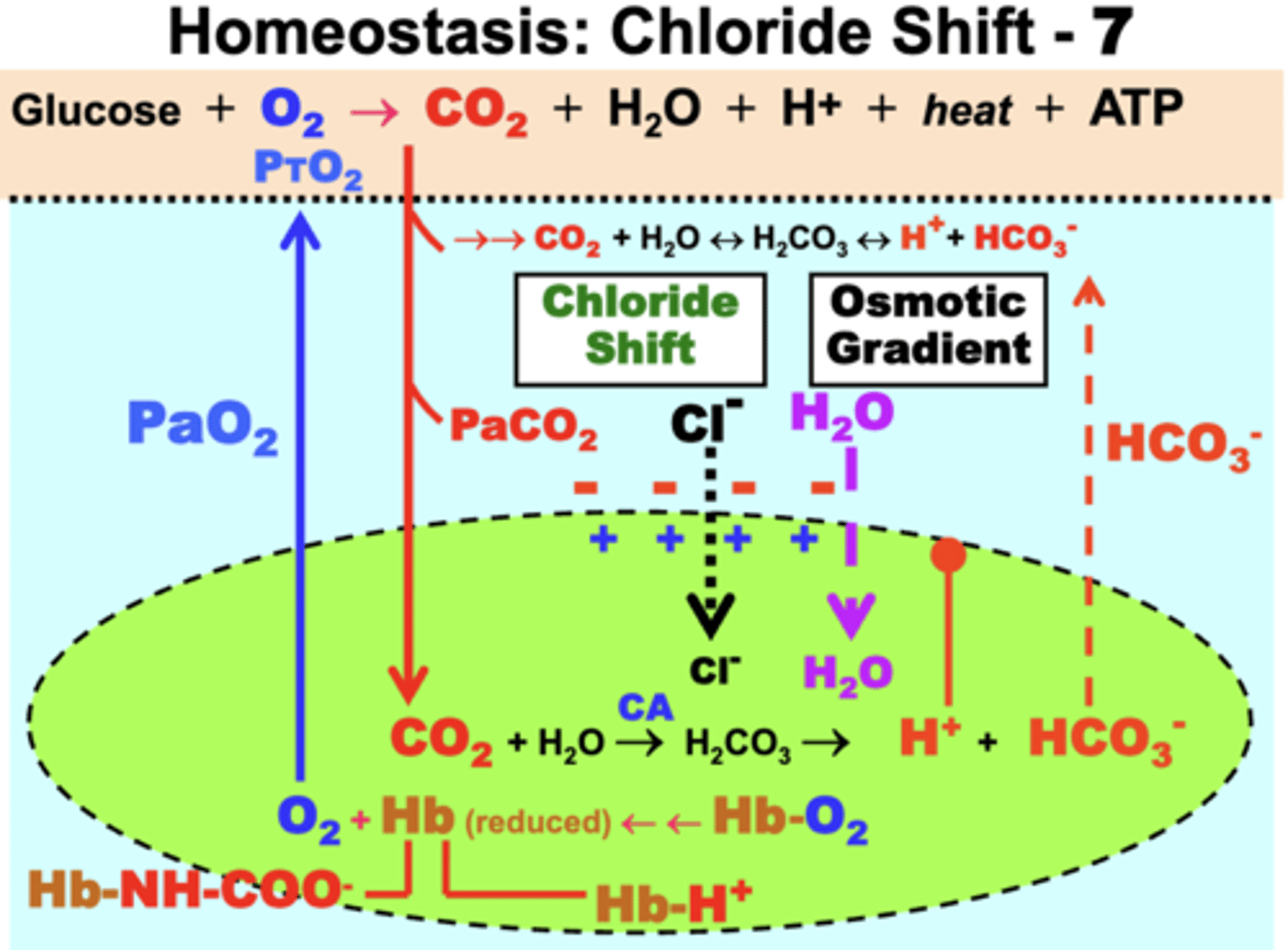
result of the Osmotic Gradient
causes H2O to enter RBCs
- RBCs tend to swell slightly as they exit tissue capillaries (venous blood)
- shrink back to size in arterial blood after effects are reversed in the pulmonary capillaries during Hb resaturation
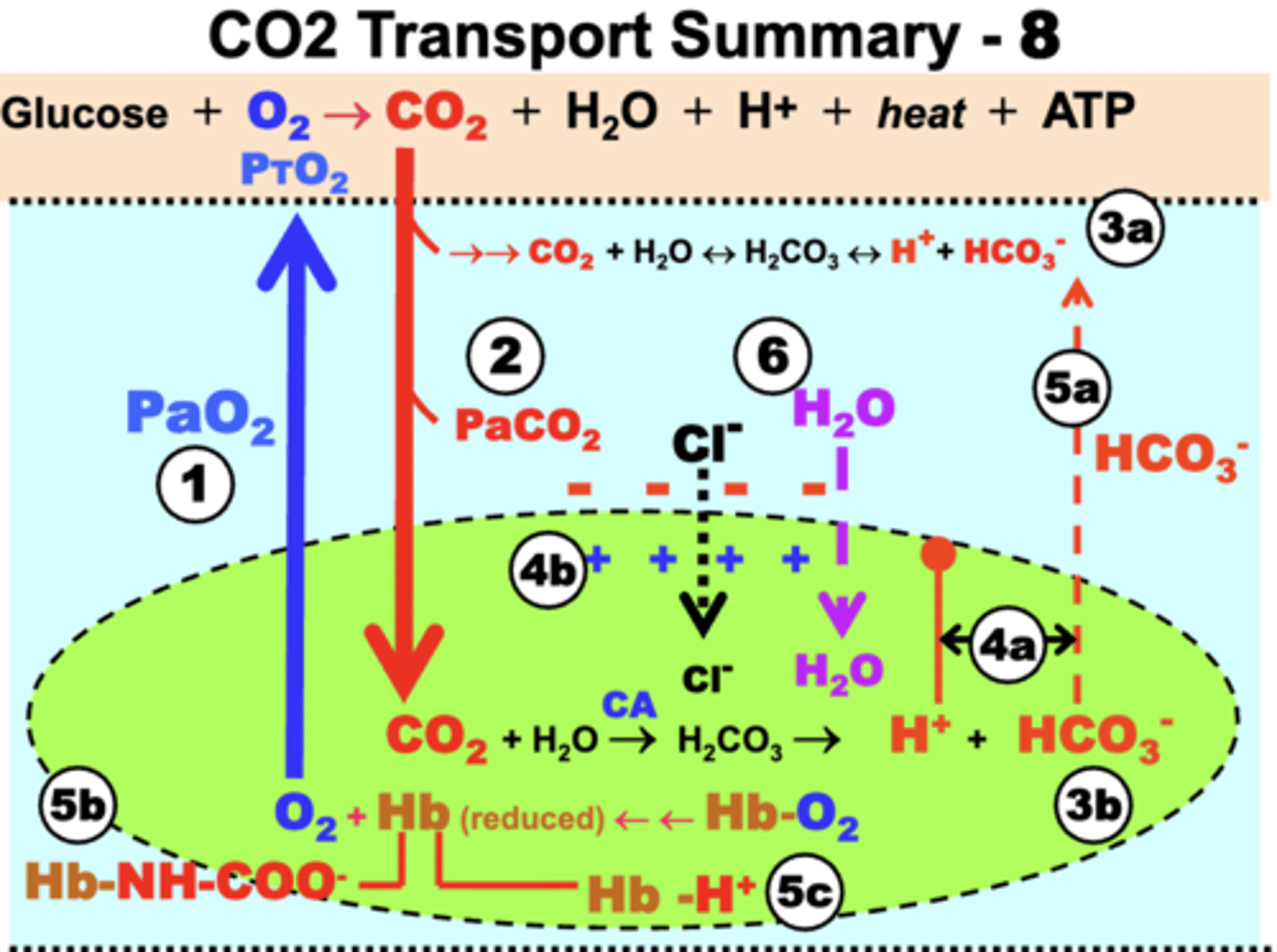
CO2 Transport Summary
1. Tissue CO2 production:
- O2 (RBC) -> O2 Tissue
- tissue oxidative metabolism -> CO2
2. Dissolved CO2 formation:
- diffusion of CO2 (tissue) -> CO2 (plasma )
3. Bicarbonate formation:
- slow hydration of CO2 (plasma) -> bicarb + H+
- rapid hydration of CO2 (RBCs) -> bicarb + H+
4. Electrochemical Gradient:
a. increased HCO3- in RBC, causes diffusion of HCO3- to plasma
b. increased H+ in RBC with limited diffusion, causes diffusion of HCO3- to plasma
5. Bohr Effect:
a. gradient causes formation of carbamino-HB in RBC, causes diffusion of HCO3- to plasma to maintain PCO2 gradient
b. carbamino-HB prevents O2 binding to deoxy-Hb
c. deoxy-Hb forms Hb-H+ to prevent rebinding of O2 and limit free H+
6. Chloride Shift:
- Cl- diffuses in from plasma to RBC = neutrality
- causes osmotic gradient = H2O flows into RBC
= reverse processes of all these will occur when venous blood returns to the lungs for reoxygenation of Hb andCO2 release into the atmosphere
CO2 transport curve
curvilinear because [CO2] is largely determined by a biochemical linear mass action relationship
![<p>curvilinear because [CO2] is largely determined by a biochemical linear mass action relationship</p>](https://knowt-user-attachments.s3.amazonaws.com/f4f6860a-3a51-4721-a778-a5f9226b6b33.png)
CO2 transport curve axes
y-axis: total [CO2] in blood (vol%)
- comprised of the sum of the gaseous/dissolved CO2, HCO3-, & carbamino compounds
x-axis: PCO2
- like O2, [CO2] is dependent on the PCO2