chem chapters 14 + 15 (carbohydrates, saccharides, monosaccharides, glucose, stereochemistry, disaccharides, polysaccharides, lipids, fatty acids, saponifiable lipids, triacylglycerols, fluid mosaic model, nonsaponifiable lipids)
1/119
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
120 Terms
life processes
biochemistry is the chemistry of ______ __________
organic; inorganic
biochemistry involves ________ and __________ molecules
empirical formula
C * H2O
examples of carbohydrates
sugar, starch
photosynthesis
energy from the sun is used by green plants to convert CO2 and water into carbohydrates
photosynthesis
what reaction is this?
6CO2 + 6H2O light energy→plant enzymes chlorophyll C6H12O6 + 6O2
6CO2 + 6H2O
what are the reactants in photosynthesis?
? light energy→plant enzymes chlorophyll C6H12O6 + 6O2
C6H12O6 + 6O2
what are the products in photosynthesis?
6CO2 + 6H2O light energy→plant enzymes chlorophyll ?
aldehydes; ketones
carbohydrates are defined as polyhydroxy __________, polyhydroxy _________ or compounds that can be hydrolyzed to them
examples of compounds that can be hydrolyzed
acetals, hemiacetals
“-ose”
carbohydrates typically end in _____
monosaccharide
simple sugars; a monomer
examples of monosaccharides
glucose, galactose, fructose
disaccharide
contains two monosaccharide units chemically united
examples of disaccharides
sucrose, maltose, lactose
what two sugars is sucrose made of?
glucose and fructose
what two sugars is maltose made of?
glucose and glucose
what two sugars is lactose made of?
glucose and galactose
polysaccharide
contains many monosaccharide units chemically united; usually these chemical bridges are made of glucose units.
examples of polysaccharides
starch, glycogen, and cellulose
what is the first function of carbohydrates?
provide and store energy
what is the second function of carbohydrates?
act as structure for cells
what is the third function of carbohydrates?
supply carbon atoms for the biosynthesis of other molecules
general formula for monosaccharides
(CH2O)n where n = 3 to 7
(CH2O)6 → C6H12O6
what would the general formula of hexose be? (CH2O)n where n = 3 to 7)
aldose
a monosaccharide that is an aldehyde derivative
ketose
a monosaccharide that is a ketone derivative
open chain; cyclic chain
equilibrium exists between two types of glucose. what are these two types?
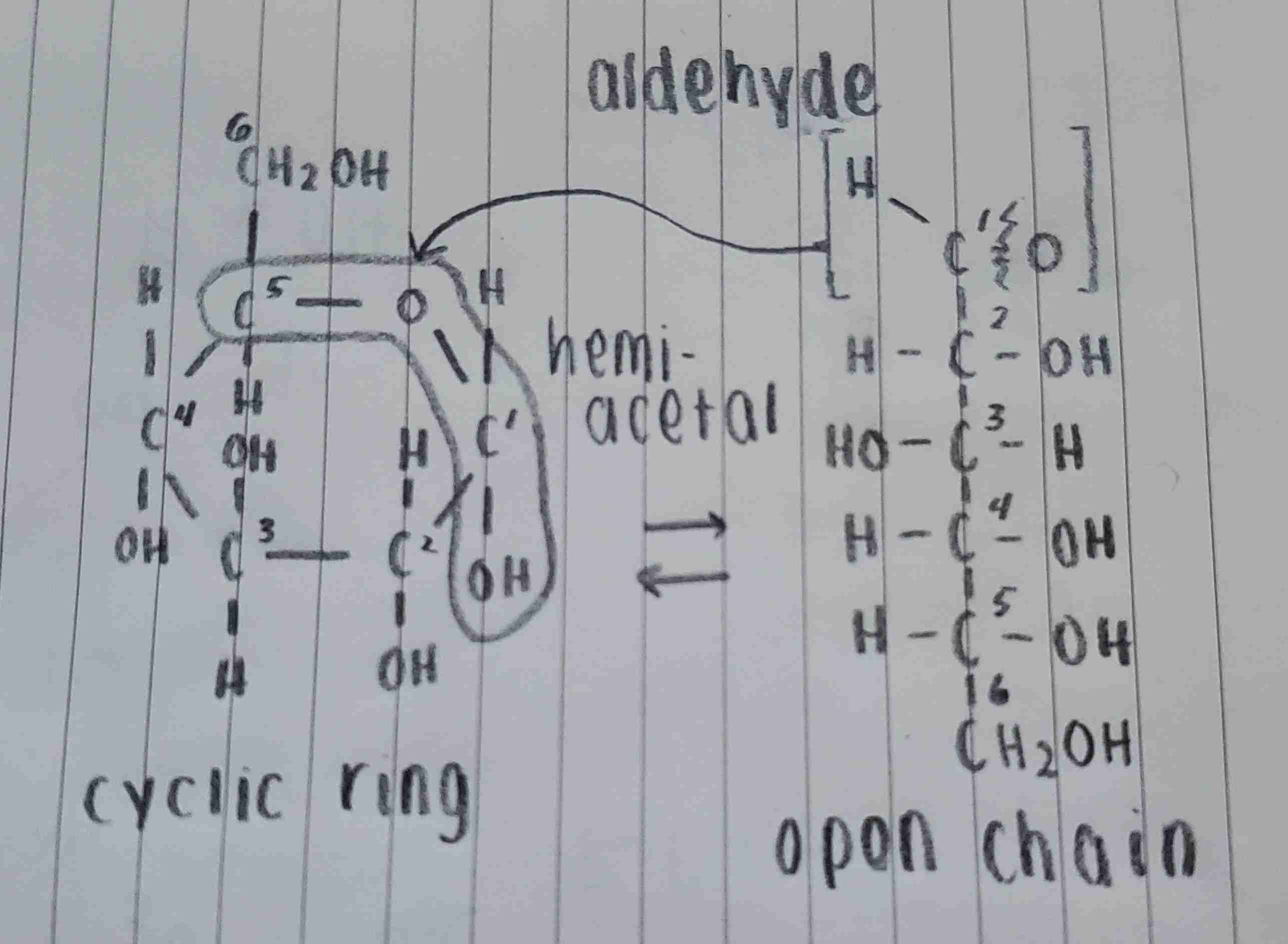
in the open chain, C1 is eletrophilic (attracts e-) and -OH on C5 is nucleophilic (donates e-). the C=O bond breaks, allowing -OH to bond with C1. this process creates a hemiacetal (C-O-C-OH) where C1 and C5 are joined by an oxygen bridge, completing the cyclic ring structure.
how does glucose go from an open chain to a cyclic ring?
oxygen
what atom joins carbon 1 and carbon 5 together in a cyclic ring?
hemiacetal
C-O-C-OH
acetal
C-O-C-O-C
up (β); down (α)
when glucose is placed in solution, the aldehyde functional group on C1 reacts with the hydroxy group on C5 which forms a hemiacetal. what are the two ways to place the hemiacetal?
anomer
a type of stereoisomer (molecules with the same formula but different 3D structures) that differ specifically in the orientation of the –OH group at the anomeric carbon (carbon 1 in glucose).
stereoisomers
compounds that have the same structural formula, however their atoms have a different arrangement in space.
enantiomers
compounds that contain at least one chiral carbon and exhibit the property of “handedness”
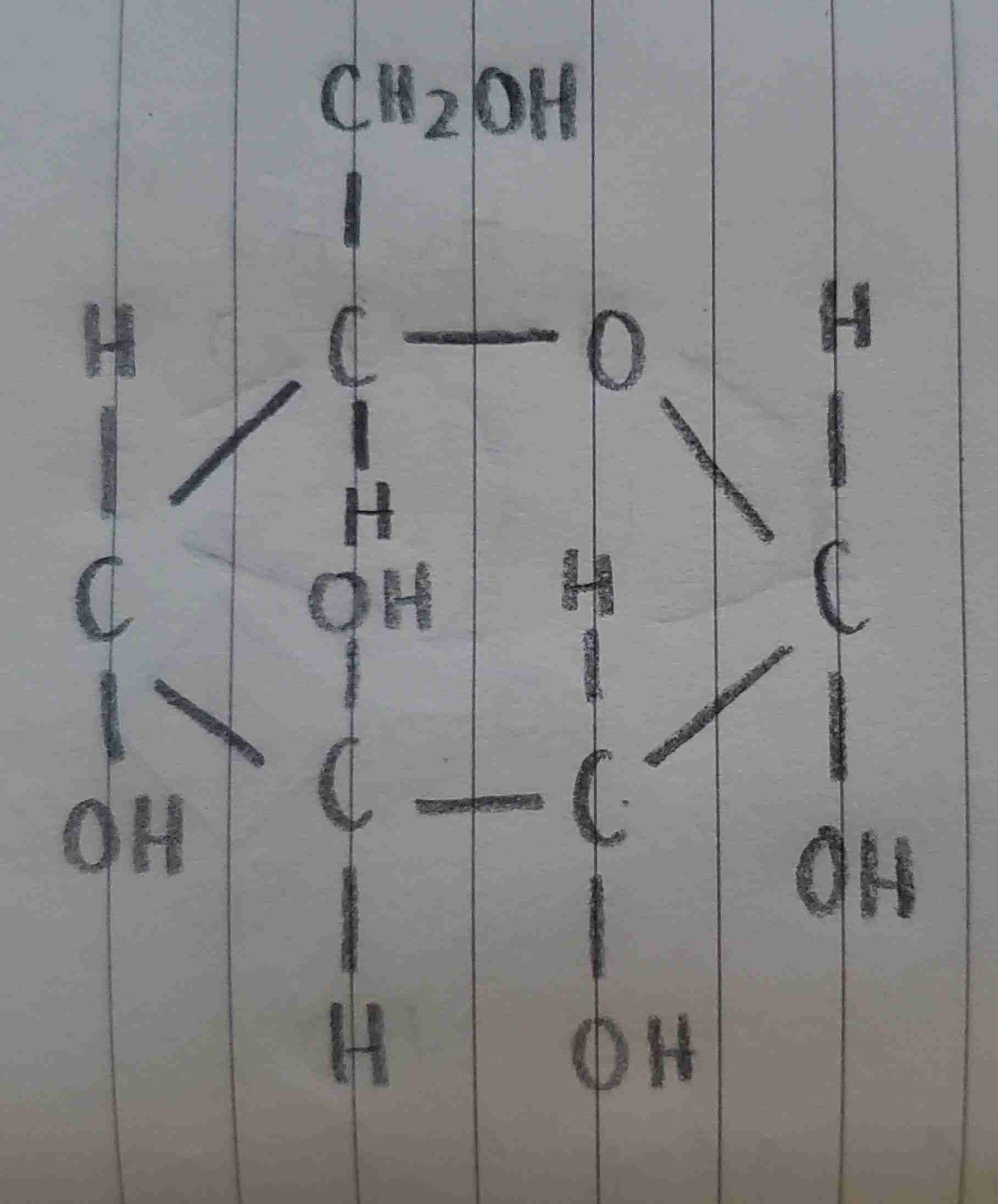
β-D-glucose
is this α-D-glucose or β-D-glucose?
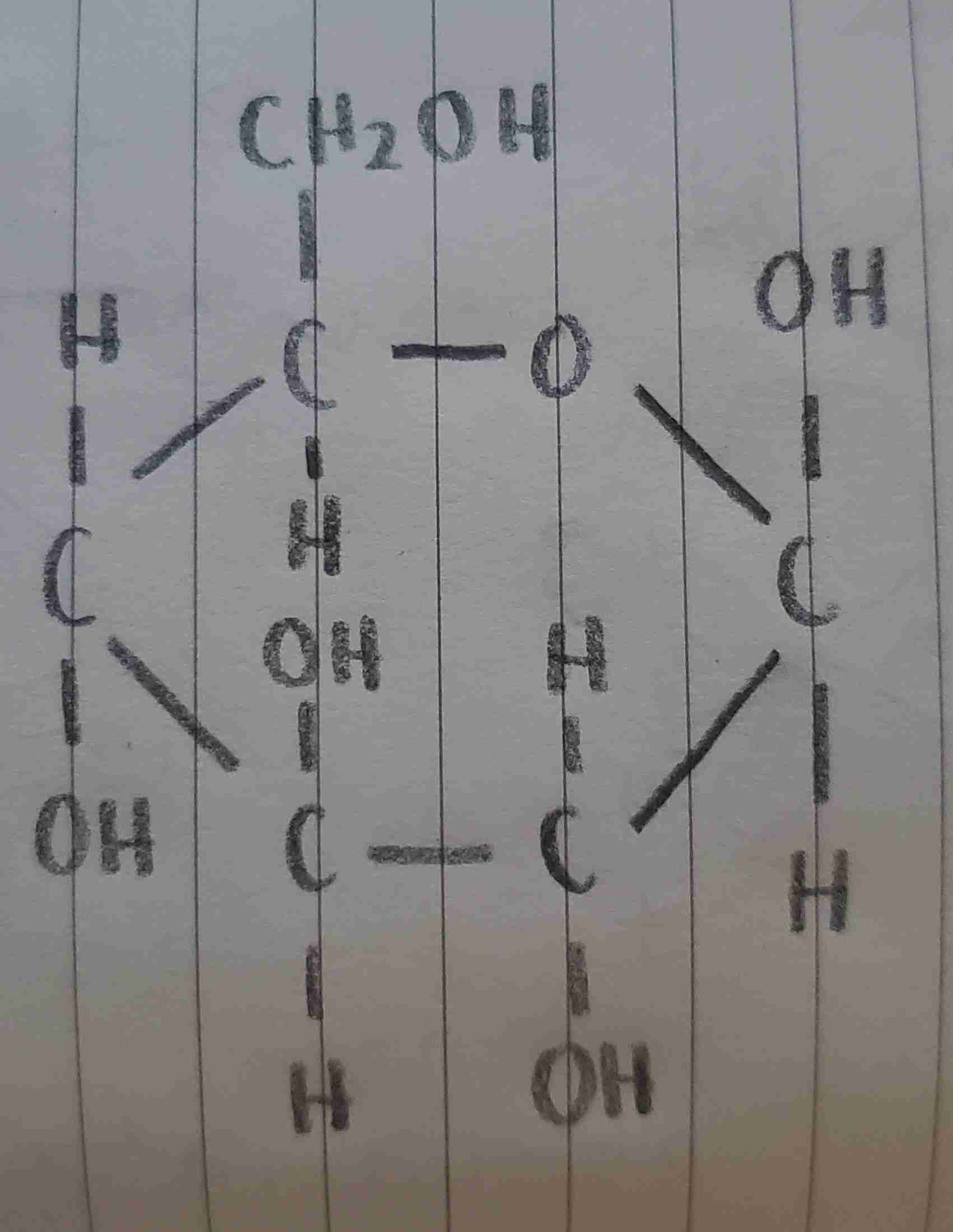
α-D-glucose
is this α-D-glucose or β-D-glucose?
anomers
α-D-glucose or β-D-glucose are what type of stereoisomers?
asymmetric; farthest
D and L classification is according to the configuration around the _____________ carbon _________ from the carbonyl carbon
right
the D classification means that the -OH group on the carbon furthest from C=O is on the _______ side
left
the L classification means that the -OH group on the carbon furthest from C=O is on the _______ side
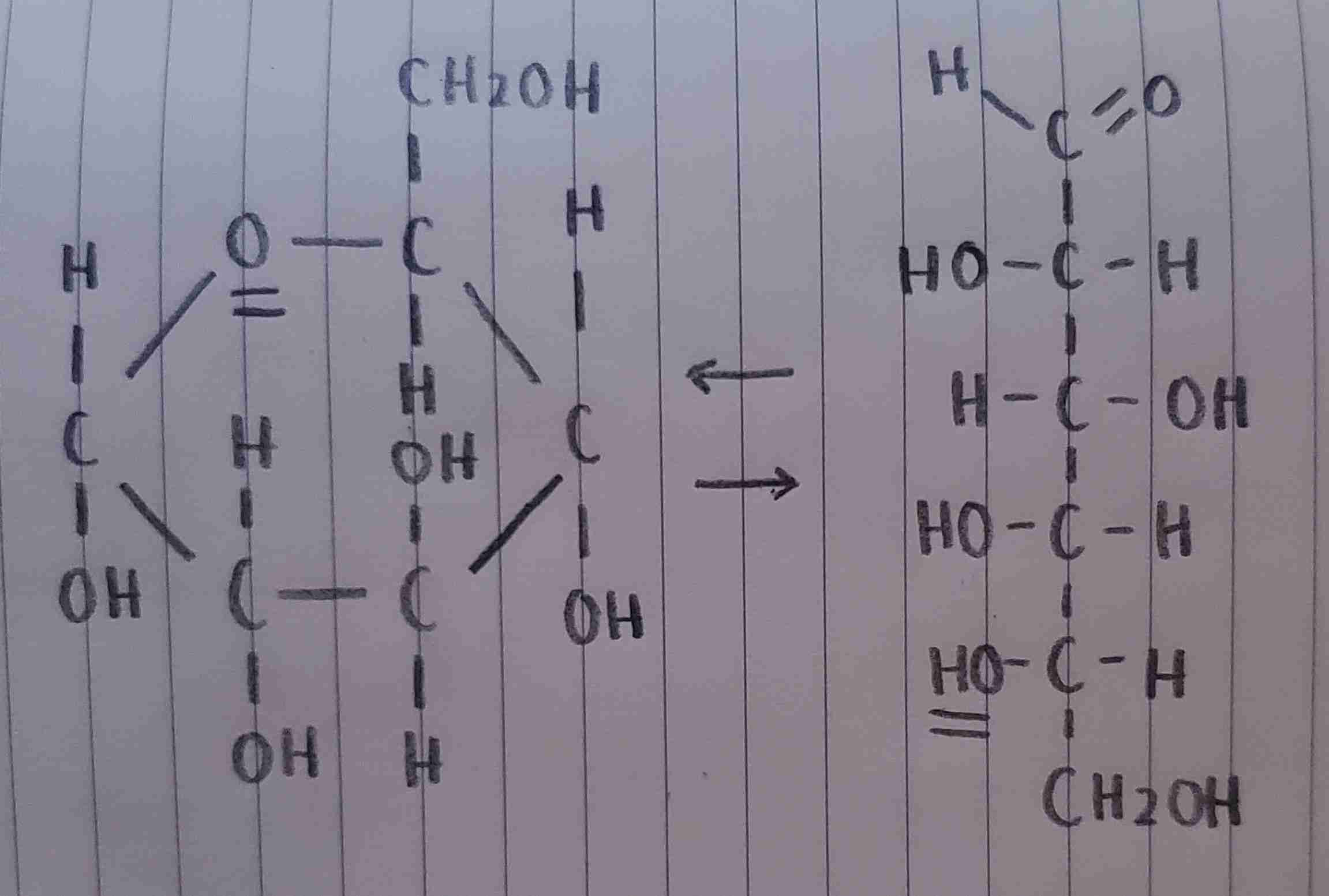
α-D-glucose
is this α-D-glucose or α-L-glucose?
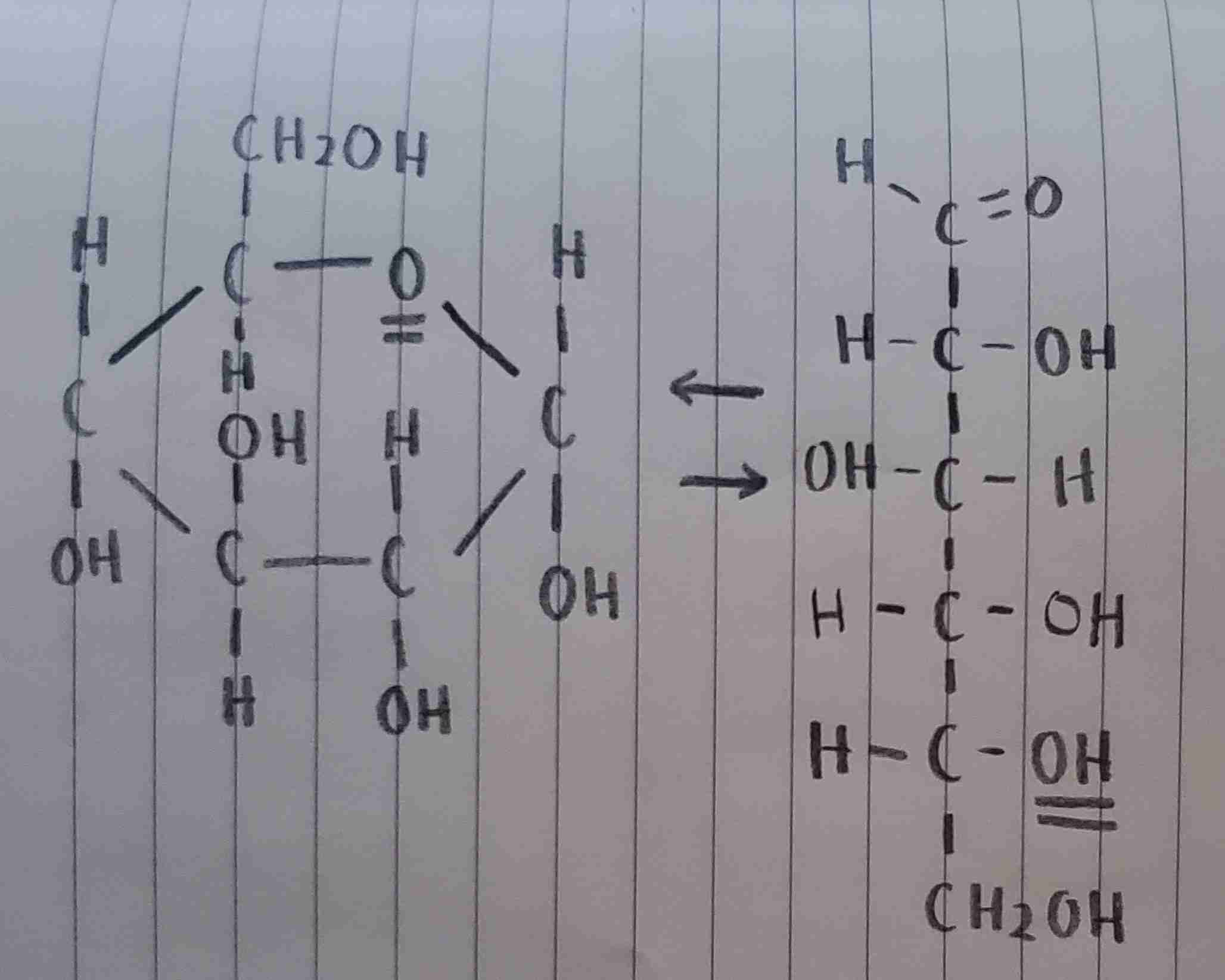
α-L-glucose
is this α-D-glucose or α-L-glucose?
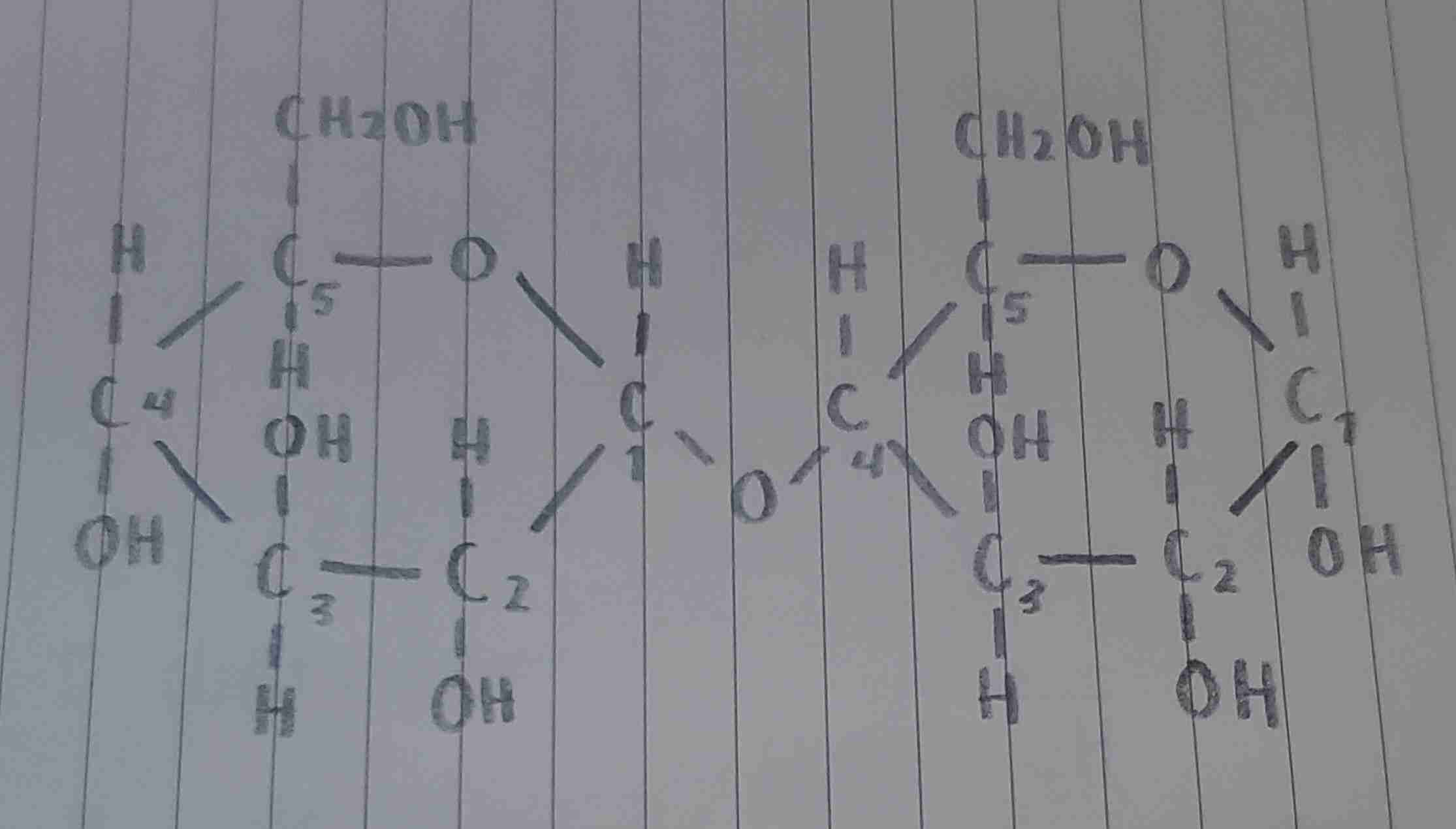
the bond that follows C5→C4 through the glycosidic linkage (C5-O-C1-O-C4)
where is the acetal located?
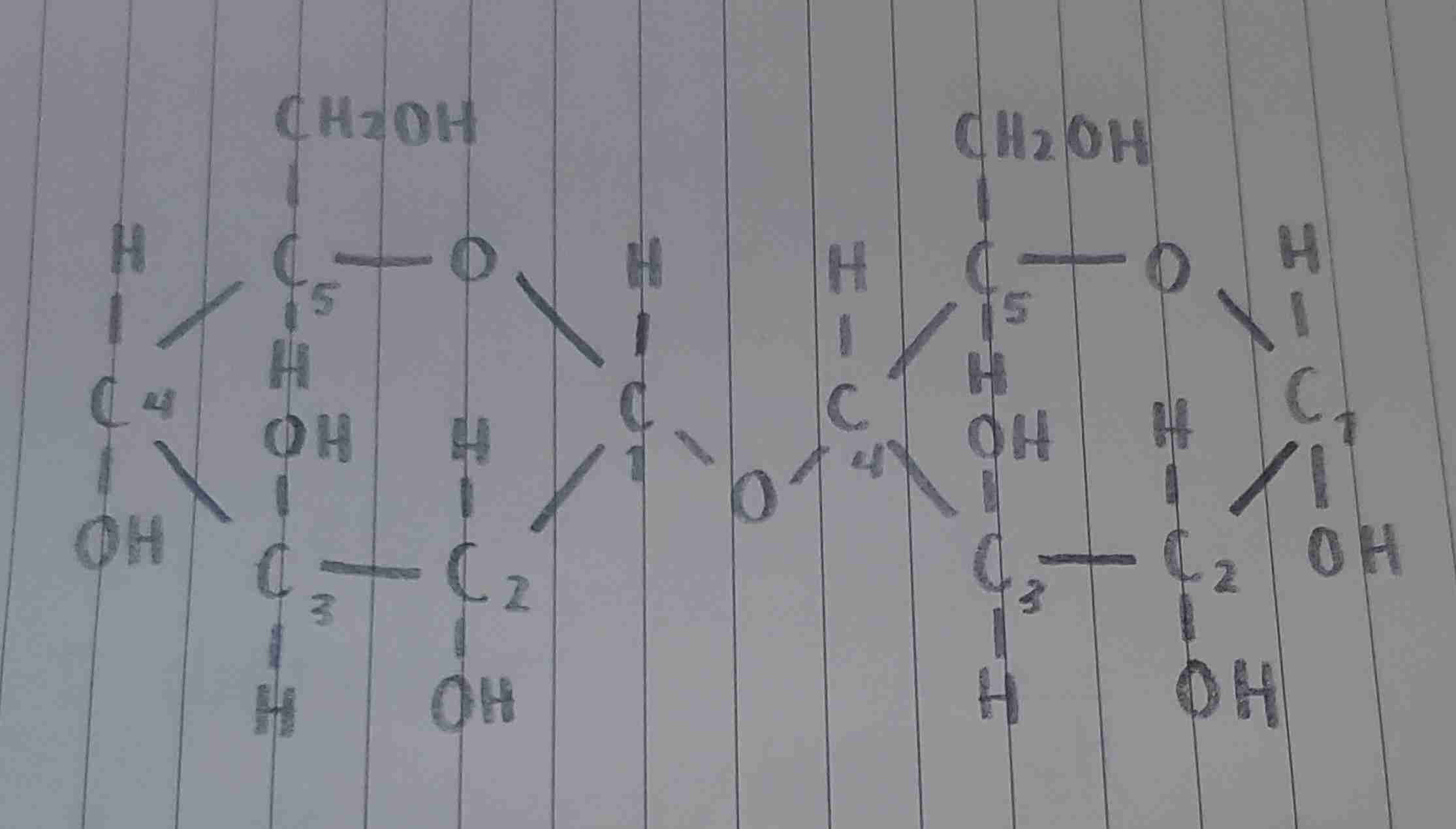
the bond where both monosaccharide units are joined (C1-O-C4)
where is the glycosidic linkage located?
down, up, down (DUD)
what -OH configuration does glucose follow?
up, up, down (UUD)
what -OH configuration does galactose follow?
Ag
reducing sugars produce ___ with Tollens’ reagent
Cu2O
reducing sugars produce ______ with Benedicts’ or Fehlings’ reagent
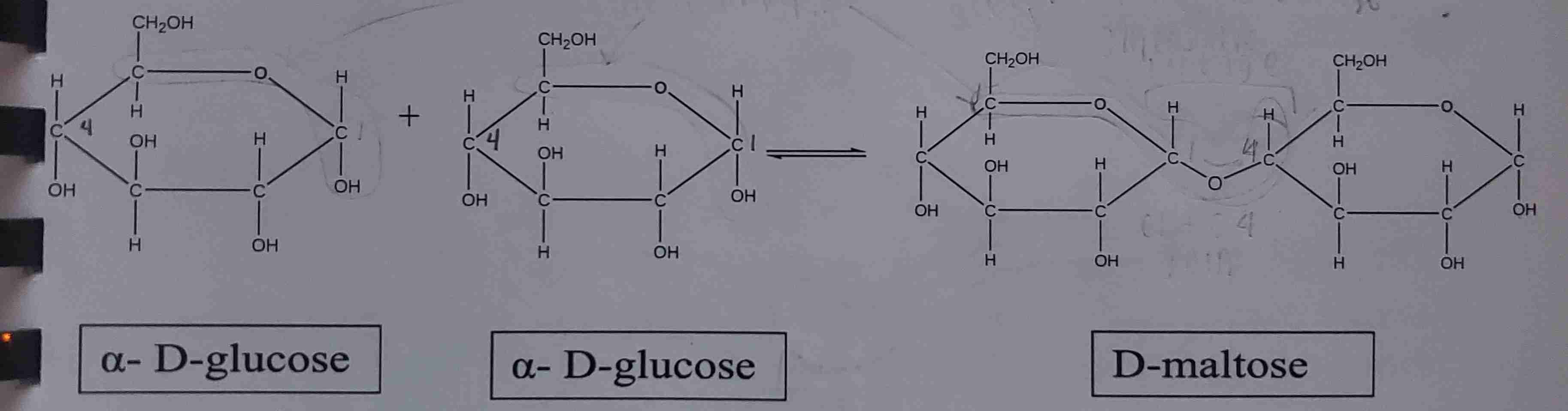
maltose
glucose + glucose → ?
glucose + glucose
? + ? → maltose
maltose formation
polymers
polysaccharides are large _________ formed from monosaccharides bonded to each other by glycosidic linkages. all polysaccharides are non-reducing sugars.
they contain an acetal which cannot be reduced
why are polysaccharides non-reducing sugars?
starch
a mixture of two kinds of bipolymers of α-D-glucose found in plants
yes
is starch found in plants?
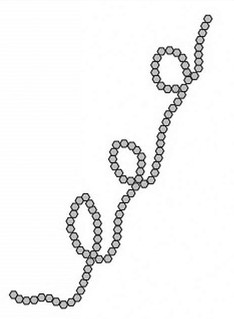
amylose (linear)
unbranched chains composed of 250 to 4000 α-D-glucose units joined by α(1→4) glycosidic linkages
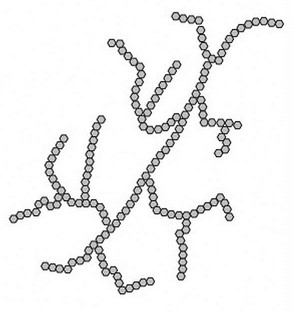
amylopectin (branched)
chains composed of more than 100,000 α-D-glucose units joined by α(1→4) and α(1→6) glycosidic linkages
amylose test
add I2 in KI solution. a positive test will show dark blue.
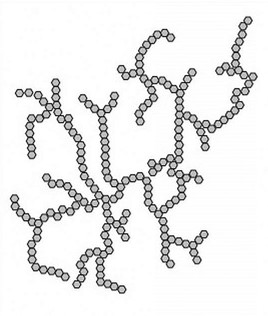
glycogen (highly branched)
resembles amylopectin except more highly branched with α-D-glucose units joined more frequently by α(1→4) and α(1→6) glycosidic linkages. it is the glucose (energy) storage carbohydrate in the animal kingdom
cellulose (linear)
the most abundant polysaccharide and a major component of the cell wall of higher plants. the β-D-glucose units are held together by β(1→4) glycosidic linkages

amylose; amylopectin
what are the two kinds of bipolymers of α-D-glucose found in plants?
lipids
substances that are insoluble in water and can be extracted from cells by a nonpolar solvent. they are greasy, nonpolar, and hydrophobic.
insoluble
are lipids insouble or soluble in water?
simple lipids
waxes, fat, oil
complex lipids
phospholipids, glycolipids, steroids
solubility
lipids are categorized based on ___________
saponifiable lipid
a lipid which contains an ester (RCO2R’) that can be hydrolyzed to yield a carboxylic acid (RCO2H) and an alcohol (ROH), or treated with a strong base to yield a carboxylate salt (RCO2-) and an alcohol (ROH)
nonsaponifiable lipid
a lipid which does not contain an ester (RCO2R’), therefore CANNOT be hydrolyzed or saponified
examples of saponifiable lipids
waxes, triacylglycerols, triglycerides
examples of nonsaponifiable lipids
steroids, terpene, testosterone
first function of lipids
structural components of membranes
second function of lipids
storage and transport forms of fuel
third function of lipids
protective coatings (cell membrane phospholipid bilayer, sebum, myelin sheath in nerves, adipose tissue that cushions organs)
fourth function of lipids
shock absorbers
fifth function of lipids
insulation
sixth function of lipids
chemical messengers
fatty acid
long hydrocarbon chains that end with a carboxyl group
saturated fatty acid
long hydrocarbon chains containing single bonds that end with a carboxyl group
terminal
the carboxyl group which is located at the end of a hydrocarbon chain in a fatty acid
general formula for saturated fatty acids
R-COOH (carboxylic acid) where R is a long unbranched chain usually with an even # of carbons
examples of saturated fatty acids
palmitic acid, stearic acid
they follow the form R-COOH and are saturated meaning they do not contain double bonds
why are palmitic acid and stearic acid categorized as saturated fatty acids?
palmitic acid formula
*follows the form R-COOH
(C16 acid)
CH3(CH2)14-COOH
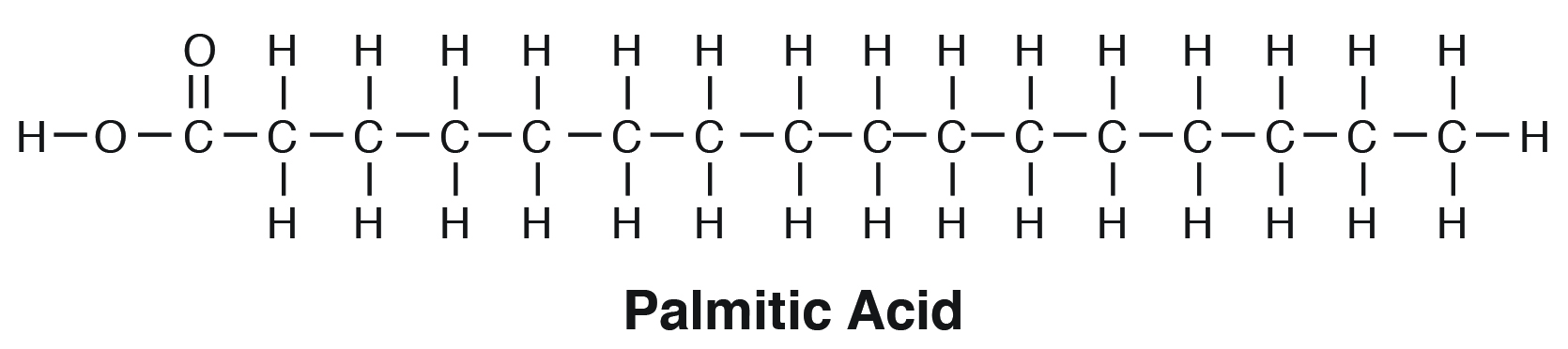
the methyl group at the end of the hydrocarbon chain
in palmitic acid, CH3(CH2)14-COOH, what does CH3 stand for?
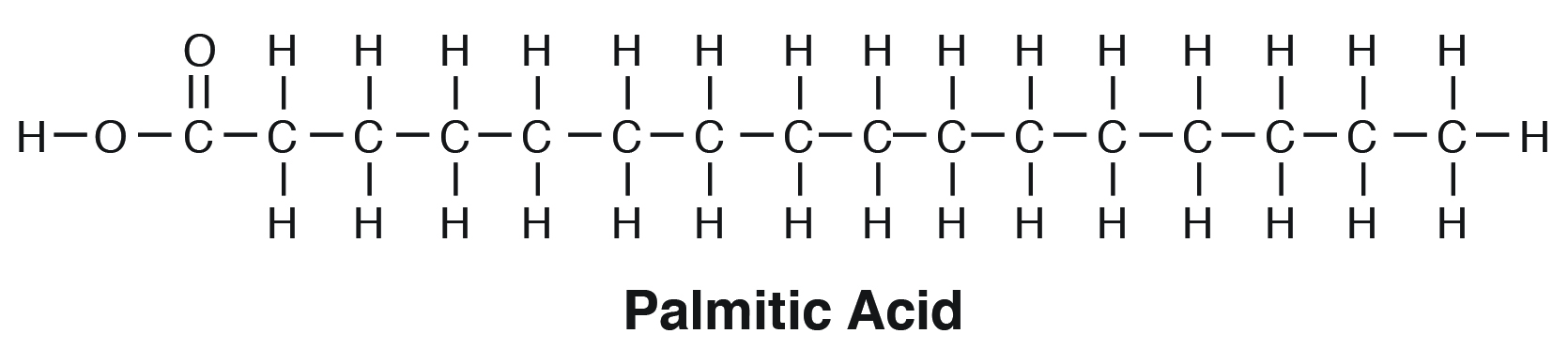
14 carbon groups
in palmitic acid, CH3(CH2)14-COOH, what does (CH2)14 stand for?
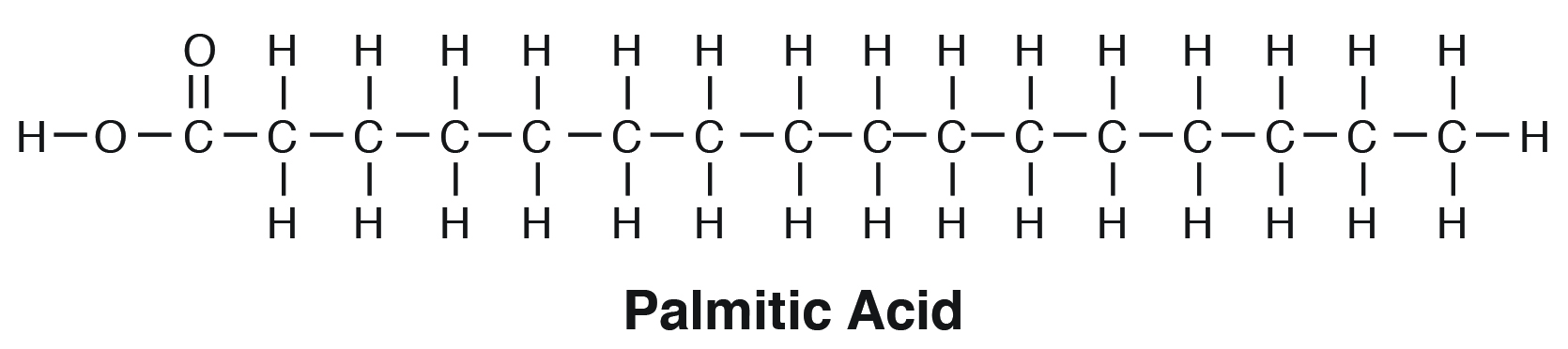
the carboxyl group at the terminal end
in palmitic acid, CH3(CH2)14-COOH, what does -COOH stand for?
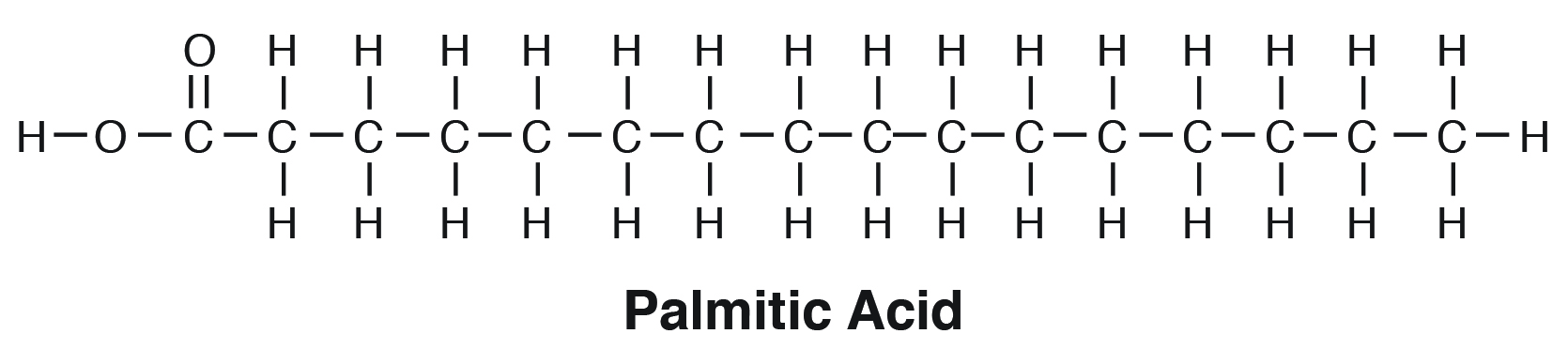
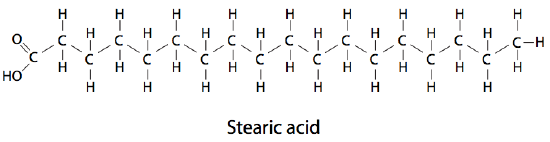
stearic acid formula
*follows the form R-COOH
(C18 acid)
CH3(CH2)16-COOH
unsaturated fatty acid
fatty acids that contain one or more double bonds in their hydrocarbon chain and end in a carboxyl group
examples of unsaturated fatty acids
oleic acid, linoleic acid
oleic acid formula
(18:1Δ9)
CH3(CH2)7CH=CH(CH2)7-COOH
monosaturated
1 double bond in the hydrocarbon chain
polysaturated
more than 1 double bond in the hydrocarbon chain
linoleic acid formula
(18:2Δ9,12)
CH3(CH2)4CH=CHCH2CH=CH(CH2)7-COOH
unsaturated fatty acid nomenclature
# of carbon:# of double bondsposition of double bonds
→ fatty acid containing 18 carbons with 1 double bond on carbon 9 → 18:1Δ9
carbon 9
where is the position of the double bond located on this fatty acid?
18:1Δ9
wax
the simplest saponifiable lipid
ester general formula
where R and R’ are long chain hydrocarbons
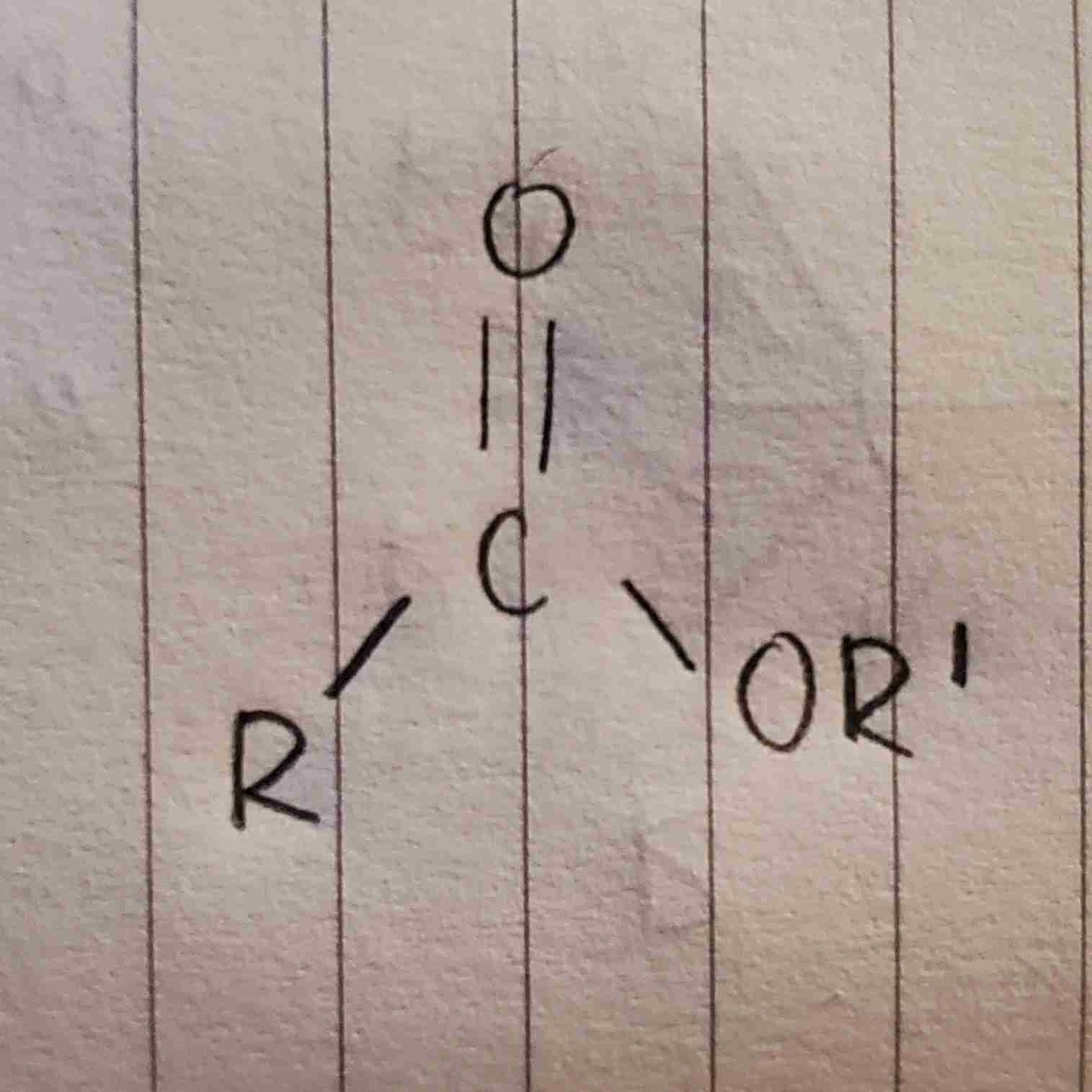
examples of waxes
beeswax, lanolin