Subatomic Particles
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
How are atoms defined?
The basic building blocks of all matter
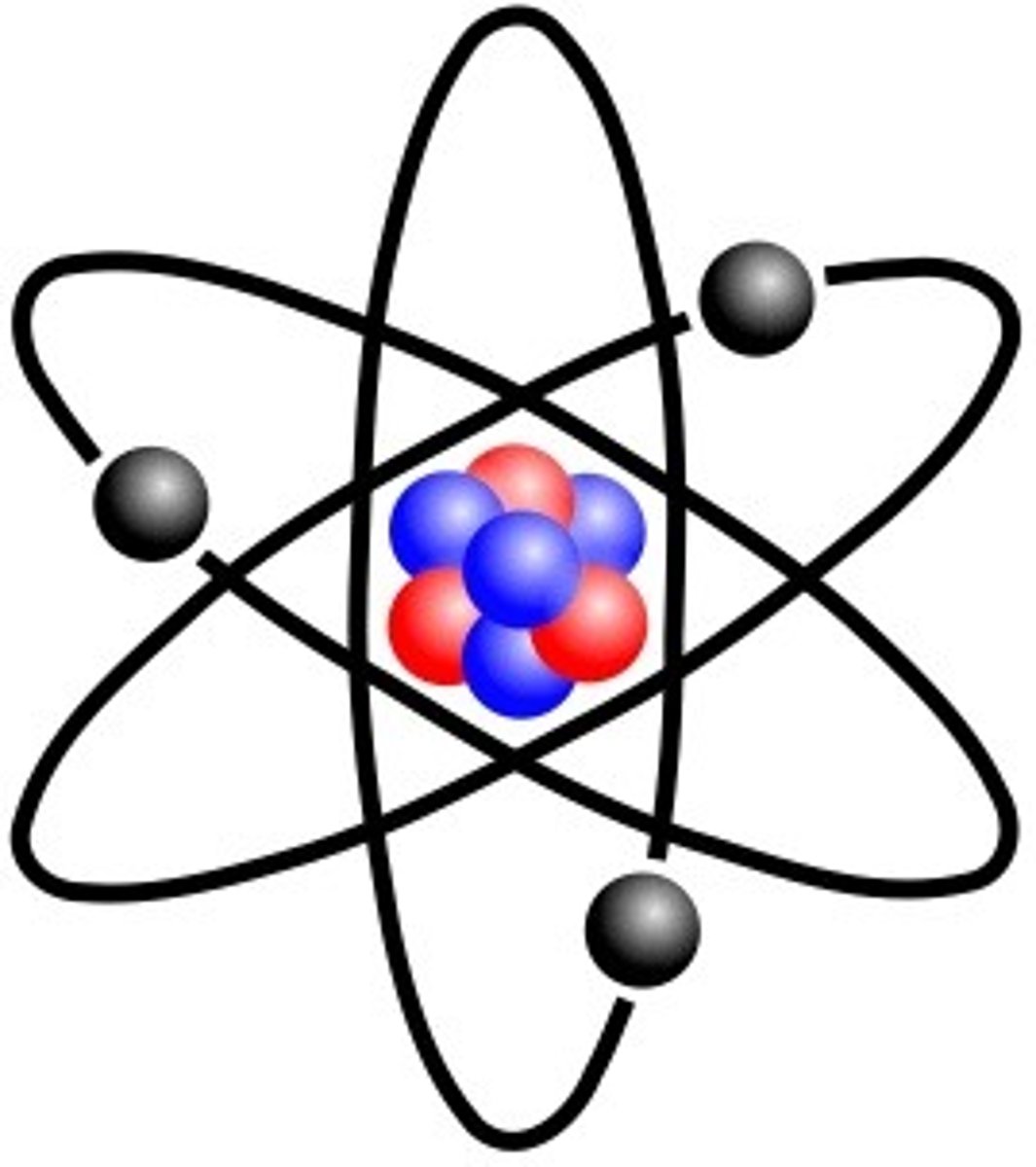
What is made up of atoms?
Elements
Nucleons
Protons and Neutrons that arein the nucleus
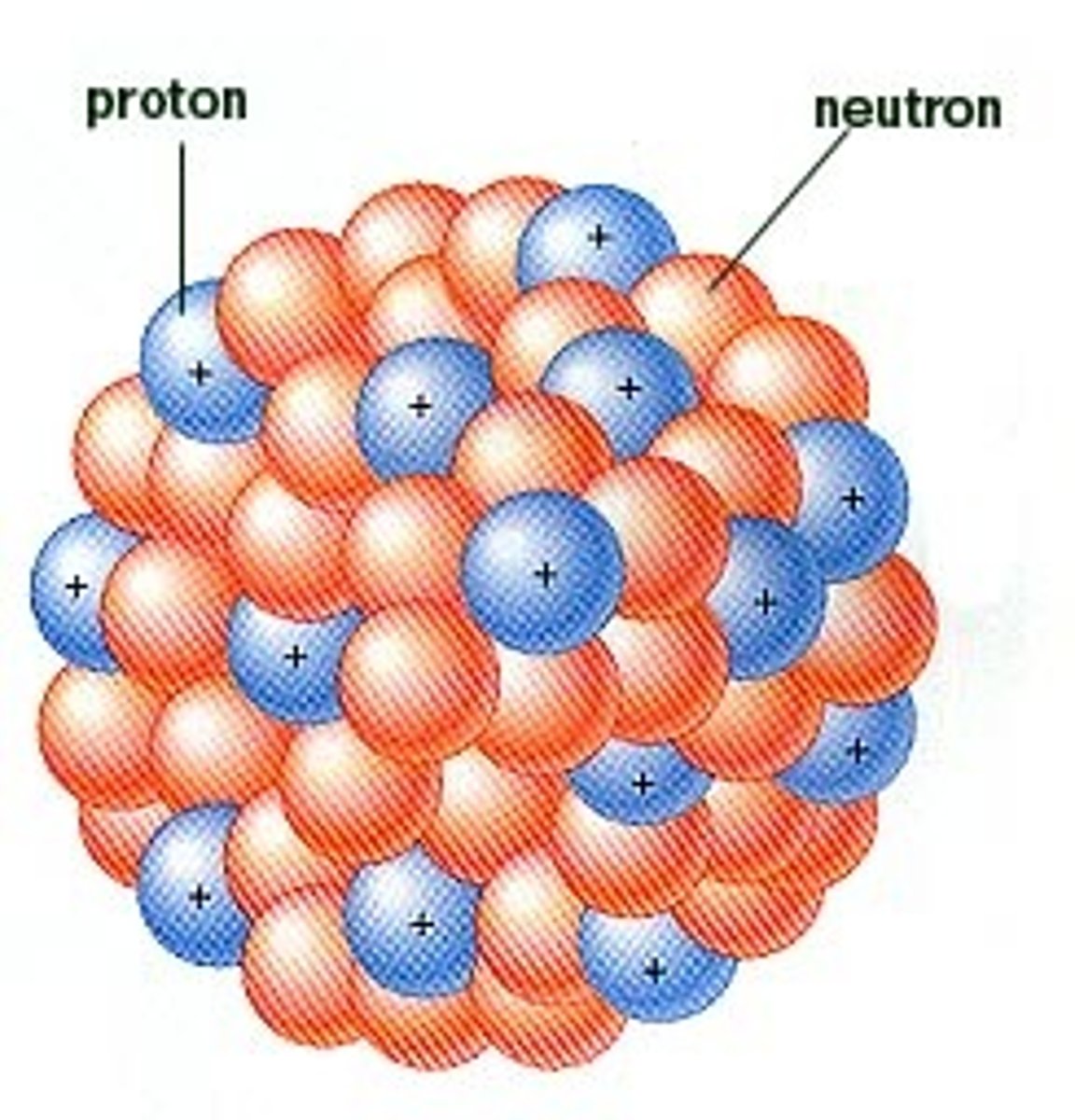
What is the atomic number always equal to?
The number of protons
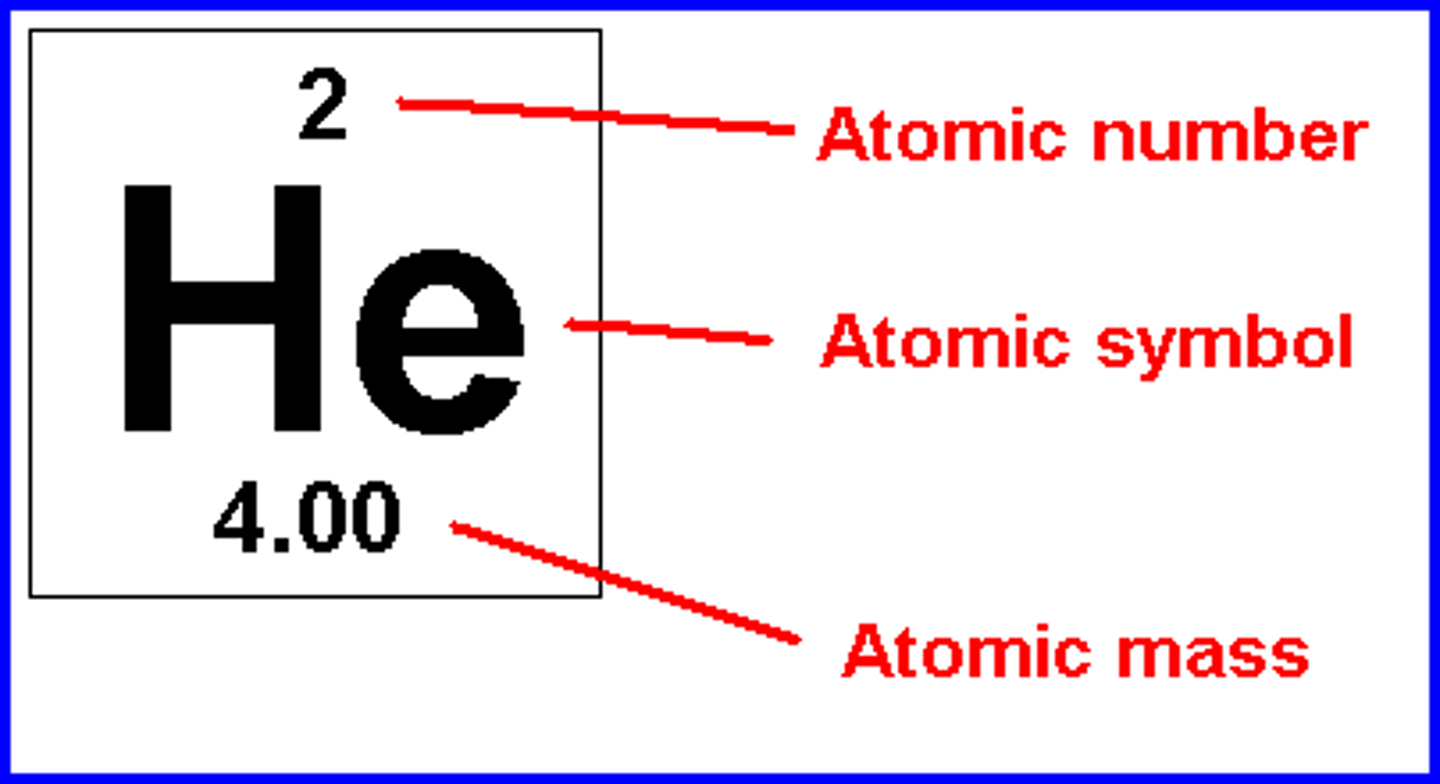
A carbon atom has:
6 protons, 6 neutrons, and 6 electrons
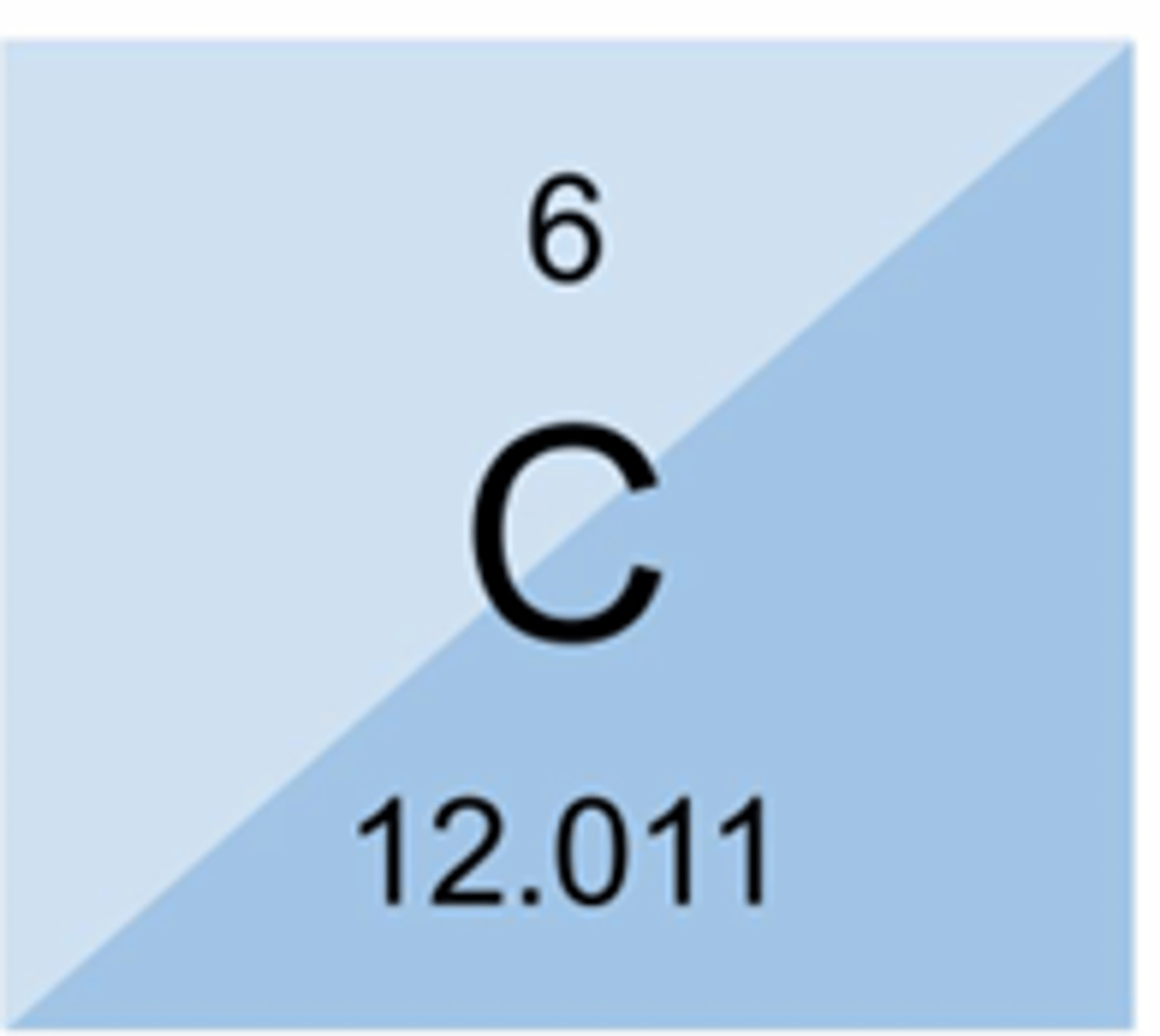
Elements are made up of...
Atoms
Protons and neutrons are...
The nucleus
The NUCLEUS has a ___________ charge
POSITIVE
"The NUCLEUS has a POSITIVE charge and is equal to the number of protons in the nucleus." It is known as what charge?
This is known as the nuclear charge.
To get the mass number,
You need to round off the atomic mass to a whole number.
The atomic number is ALWAYS EQUAL to...
the number of protons.
-The atomic number is ALWAYS equal to the number of protons
How many protons does a carbon atom have? __________