Carboxylic Acids
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
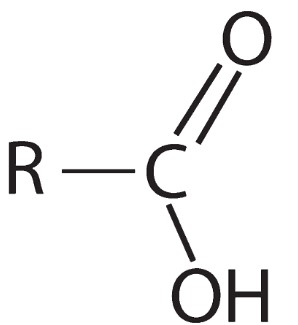
What are carboxylic acids?
A homologous series containing -COOH as its functional group.
How can carboxylic acids be formed?
Carboxylic acids can be formed by the oxidation of alcohols using acidified dichromate(VI) H+/Cr2O7.
How are carboxylic acids named?
It is named by replacing the ‘-e’ from the name of the main alkane chain with ‘-oic acid’. The carbonyl carbon is counted as part of the main carbon chain and is the first carbon of the chain.
“Carboxylic acids are weak acids.” Explain what this means.
They are weak acids, so only partially ionise in aqueous solution.
What is formed when carboxylic acids react with metals?
Salt + hydrogen gas.
What is formed when carboxylic acids react with bases / alkalis?
Salt + water.
What is formed when carboxylic acids react with carbonates / hydrogen carbonates?
Salt, carbon dioxide + water.
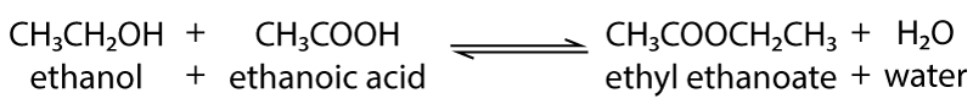
What is esterification?
When a carboxylic acid reacts with an alcohol in the presence of an acid catalyst (usually conc. sulfuric acid, H2SO4).
In the reaction, the OH bond in the alcohol is broken.
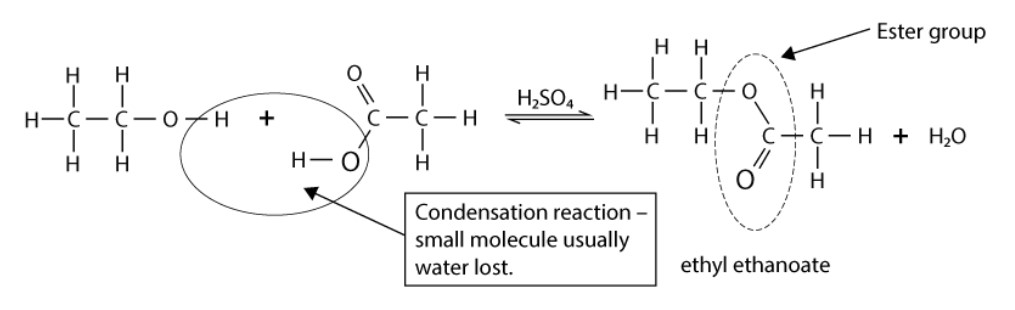
What products are formed when a carboxylic acid reacts with an alcohol?
An ester + water.
State one characteristic of the ester formed.
Ester produced has a sweet, fruity smell.
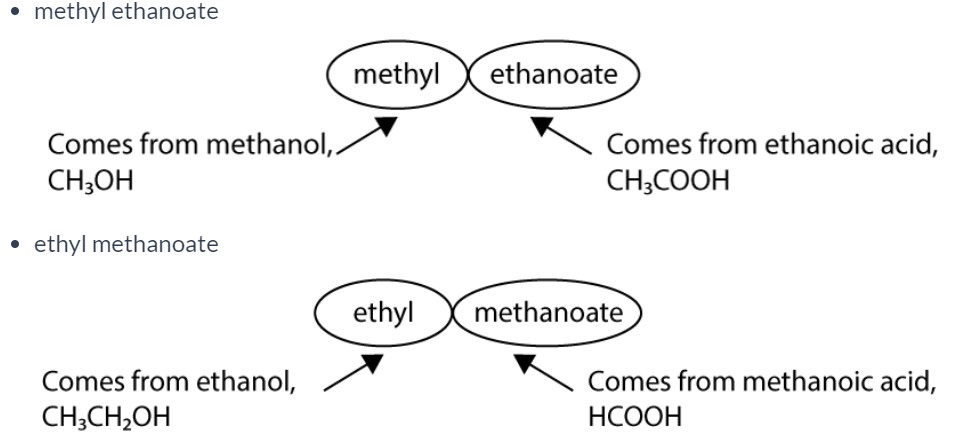
How are esters named?
The name of an ester is derived from the names of the two reactants from which it formed. The first part of the name always relates to the alcohol and the second part of the name relates to the carboxylic acid.
What conditions are needed for an esterification reaction?
Heat the reactants under reflux with an acid catalyst (usually sulfuric acid).
Explain how you would increase the yield of the ester produced, using Le Chatelier’s Principle.
In order to have a good yield, the reaction flask is attached to a distillation set up so the ester can be removed as soon as it is formed.
In doing this ,Le Chatelier’s principle states that the position of equilibrium will shift to the right to replace the ester, and so more product is formed.