Chemistry - 2.5 Crude Oil, Fuels and Organic Chemistry
1/48
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
What is crude oil - look, formation, atomic structure
Thick, black viscous liquid that floats on water.
It was formed from the remains of sea animals and plants that died and were buried millions of years ago and under high pressure and temperature, formed crude oil.
A mixture of hydrocarbons with different chain lengths.
What is a hydrocarbon?
A molecule that contains only carbon and hydrogen.
What must happen to crude oil before it is used?
Must be separated into fractions through fractional distillation.
What are the 4 steps of fractional distillation?
The crude oil is heated until it is vaporised (boiled).
The gaseous crude oil enters the bottom level of a fractional distillation column, where the temperature is highest.
Hydrocarbons that have a boiling point higher than the temperature of the level they are on condense into a liquid and are piped away.
The hydrocarbons with boiling points lower than the temperature of that level remain as a gas and travel up to the next level, which is cooler.
As hydrocarbon chain length increases, what happens to the properties of the fractions? (5)
Become darker in colour (colourless to yellow brown)
Higher boiling points
Become more viscous
Burn dirtier
Become harder to ignite.
Order of the 7 key fractions in a fractional distillation tower with their temperatures. (top to bottom)
Petroleum gases - 20°
Petrol - 70°
Naphtha - 120°
Parafin (kerosene) - 180°
Diesel oil (gas oil) - 260°
Lubricating oil - 300°
Bitumen
Uses of the following:
Petroleum gases
Petrol
Naptha
Paraffin (kerosene)
Diesel oil (gas oil)
Lubricating oil
Bitumen
Gas stoves
Cars
Chemicals, such as plastic
Jet fuel
Diesel engines
Reducing friction in engines and gear
Asphalt (roads)
What happens to the longest chains in a fractional distillation tower?
They condense towards the bottom of the tower.
What happens to the shortest chains in a fractional distillation tower?
They rise to the top.
Why do properties change as the chain length gets longer?
As the hydrocarbon chains get longer, the intermolecular forces become stronger. This means that more energy is needed to overcome them in order for melting or boiling to occur in longer hydrocarbon chains. These forces also explain why longer chain hydrocarbons are more viscous.
Economic issues and benefits of crude oil production. (2)
Countries that produce crude oil set the price, and since it is essential to the economy, the oil-purchasing economies have to pay. This is detrimental to poorer countries and oil-purchasing countries have less control on their economy.
Any major problems in oil-producing countries (such as war, natural disasters or political upheaval) can have a major impact on the global economy.
The crude oil industry provides a lot of jobs, which is of great help to the economy.
Environmental issues of crude oil production. (2)
Burning fuels from crude oil releases carbon dioxide into the atmosphere. Higher carbon dioxide levels in the atmosphere have been linked to global warming, a major contributor to climate change.
Crude oil can leak into the environment when it being pumped from the ground or being transported around the world in ships.
What happens when a fire burns?
A combustion reaction.
When does a combustion reaction occur?
When a chemical reacts with oxygen to produce heat (exothermic), light and new products.
What is the equation for the combustion of hydrocarbons?
methane + oxygen → carbon dioxide + water
CH4 + 2O2 → CO2 + 2H2O
Equation for the combustion of alcohol?
ethanol + oxygen → carbon dioxide + water
CH3CH2OH + 3O2 → 2CO2 + 3H2O
Combustion of hydrogen equation.
hydrogen + oxygen → water
2H2 + O2 → 2H2O
2 advantages of hydrogen as a fuel.
It is a renewable resource as it is made from electrolysis of water.
It does not contribute to global warming as it does not release carbon dioxide.
3 disadvantages of hydrogen as a fuel.
Large amounts of electricity are required to produce it from electrolysis of water (and most electricity is still produced by burning fossil fuels).
Bulky and heavy storage containers are required to contain the gas.
It forms a flammable and potentially explosive mixture with air.
What is the fire triangle.
Contains the three main components of a fire. When any one of the three components (heat, fuel or oxygen) is removed, the fire is extinguished.

What is the main method to remove heat from a fire?
Add water. Most suitable for fires when the fuel is solid (e.g. paper, wood, coal, etc.)
What are 3 methods for removing oxygen from a fire?
CO2 extinguishers
foam extinguishers
fire blankets.
These methods are most suitable for fires that water is not suitable for such as electrical fires and liquid fuel fires (e.g. chip pan fires and aircraft fuel fires).
3 methods of removing the fuel in fire prevention?
managed forests having sections with no trees to prevent forest fires from spreading – this is known as a firebreak
felling and removing trees around a fire (if there is time) to create a firebreak and stop the fire from spreading.
use of non-flammable or fire-resistant materials in the manufacture of everyday items such as: clothing, furniture, and the walls, ceilings and doors of buildings.
What is the method for finding the energy released per gram of fuel burned? (8)
Place the fuel in a spirit burner.
Weigh the spirit burner and fuel.
Measure 100cm3 of water into a conical flask.
Measure the temperature of the water, ensuring the thermometer does not touch the bottom of the flask.
Use the spirit burner to heat the water in the flask for 5 minutes.
Measure the temperature of the water to calculate the temperature rise.
Weigh the burner and fuel again once it has cooled to calculate the mass of fuel used.
Calculate the energy released by the fuel.
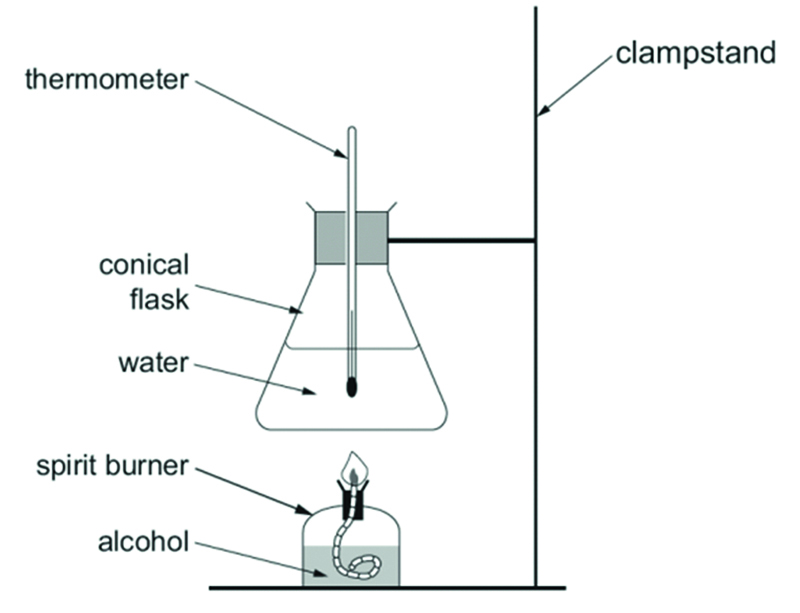
How do you calculate the energy released by the fuel in the specified practical?
Energy released (J/g)=
mass of alcohol burned x mass of water × 4.2 × temperature rise
mass of alcohol burned
How to improve the specified practical? (3)
Enclose apparatus or reduce drafts as it would mean less heat is lost between the flame and the conical flask.
Use a copper calorimeter instead of a glass conical flask as it is a better conductor, so more heat is transferred to the water.
Put a lid on the copper calorimeter because it improves insulation so less heat is lost after transfer to water.
What is crude oil mainly made of?
Alkanes.
What is an alkane?
Alkanes are hydrocarbons that contain carbon and hydrogen atoms with only single bonds. They are also known as saturated hydrocarbons.
What’s the general formula for an alkane?
CnH2n+2
2 Steps to name alkanes and alkenes
The first part depends on the number of carbons
Second part is ‘ane’ if single bond, and ‘ene’ if double bond.
First part of name for the first 10 hydrocarbon compounds.
Meth
Eth
Prop
But
Pent
Hex
Hept
Oct
Non
Dec
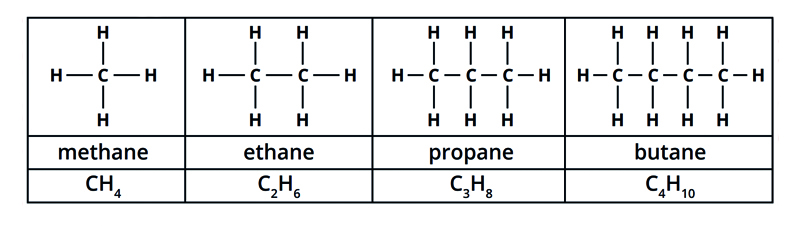
What are the names, formulae and structural formulae of the first four alkanes?
Methane - CH4
Ethane - C2H4
Propane - C3H8
Butane - C4H10
What is cracking used for?
Most of the alkanes from crude oil have long carbon chains. Long chain alkanes are not as useful as shorter chain alkanes (due to them not being useful as fuels); therefore, cracking can be used to form shorter chain alkanes from long chain alkanes.
What does cracking involve?
Cracking involves heating the long chain alkanes in the presence of a catalyst but in the absence of oxygen, which breaks them down into smaller hydrocarbons.
What are the product of cracking?
Shorter chain alkanes (which are more useful as fuels) and an alkene.
What are alkenes?
Alkenes are hydrocarbons that contain a carbon-carbon double bond in the carbon chain. They are also known as unsaturated hydrocarbons.
What is the general formula of alkenes?
CnH2n
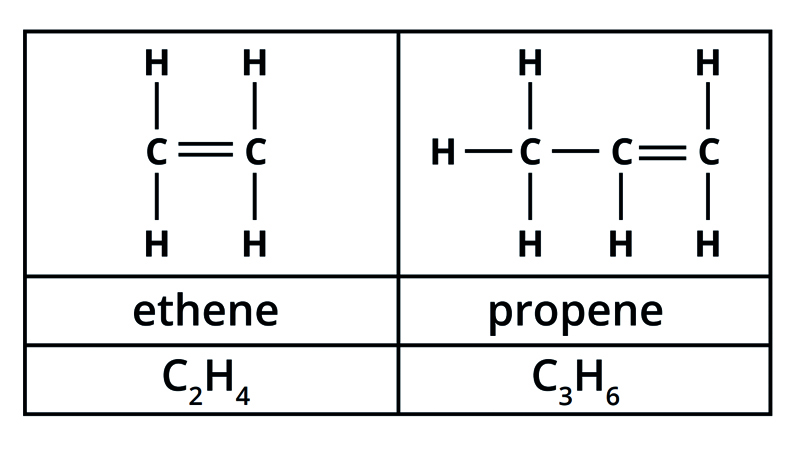
What is the name, formulae and structural formulae of the first two alkenes?
Ethene - C2H4
Propene - C3H6
How does having a double bond change the chemistry of a hydrocarbon?
The double bond makes alkenes more reactive than alkanes.
What is an addition reaction?
This is where a small molecule is added to a C=C double bond, leaving a single bond in its place. The atoms of the small molecule attach to the carbon atoms in the alkene.
Addition of Br2 to an alkene?
A good test for alkenes, as the orange bromine turns colourless as the reaction happens. The example below shows the addition of Br2 to ethene.
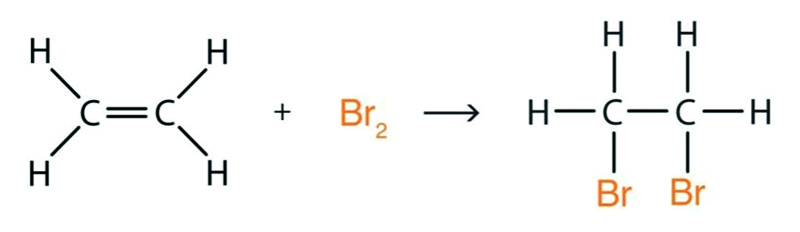
Addition of H2 to alkenes?
This requires a nickel catalyst and is commonly known as hydrogenation. It is used in the production of margarine. The example below shows the addition of H2 to ethene.
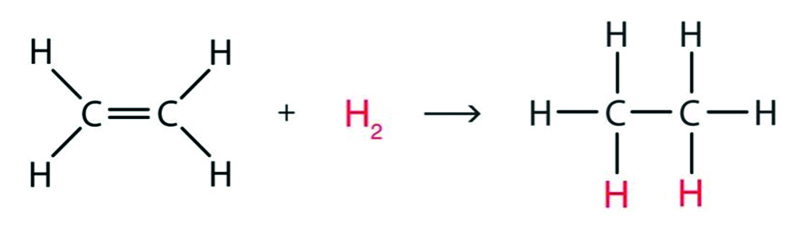
What is polymerisation?
Alkenes can also be used to make polymers (long chain molecules of carbon).
What is a monomer?
Another name for an alkene. It is a small reactive molecule that, when joined together with others of its kind, forms a polymer.
During polymerisation, what happens?
One of the bonds in the double bonds breaks and then joins onto another monomer. This process repeats until a long polymer chain is formed.
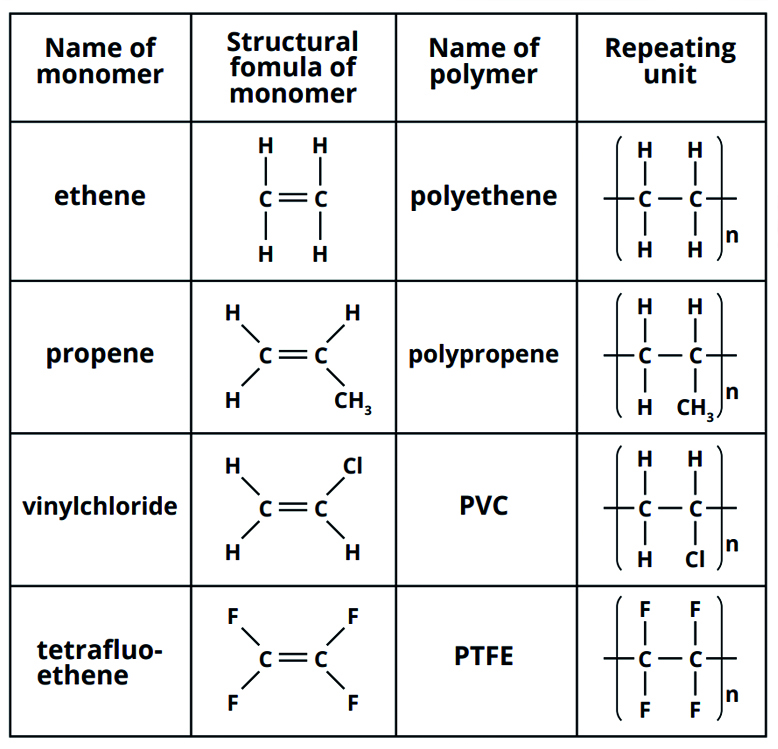
What is the structure, name, repeating unit, and uses of the following 4 monomers?
Ethene
Propene
Vinyl Chloride
Tretrafluroethene
Polyethene - plastic bags and bottles
Polypropene - ropes and plastic crates
PVC - drainpipes and window frames
PTFE - non-stick coating for pans
Why is disposing of plastic waste problematic? (2)
Disposing of plastic in landfills is not effective as landfills quickly fill up and plastic waste will not decompose for hundreds of years as it is not biodegradable and is highly resistant to most chemical reactions.
Burning plastics is not desirable as they release carbon dioxide as they burn (contributing to global warming). They also release a number of toxic gases as they burn.
Why is recycling plastic good? (3)
Recycling plastic is the most effective solution to this problem as it reduces the amount of plastic waste that is going to landfills or being burned.
Recycling helps conserve crude oil reserves, as less new plastic is required.
Finally, recycling uses less energy than producing new plastics and so fewer fossil fuels need to be burned.
What is an isomer?
This is when the main carbon chain (sometimes known as the parent chain) of a molecule is shortened and extra carbon and hydrogens are present as a ‘branch’ from the main chain.