Functional groups and reactivity
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
what are functional groups?
-sets of atoms with distinct chemical properties
-independent of the rest of the molecule
-specific reactivity and interactions with other molecules
how are functional groups normally distinguished?
by the heteroatoms they contain and the oxidation level (e.g., alcohol vs aldehyde for oxygen)
what are the four oxidation levels for carbon-oxygen functional groups?
-one bond: alcohol oxidation level
-two bonds: aldehyde oxidation level
-three bonds: carboxylic acid oxidation level
-four bonds: carbon dioxide oxidation level
what are alcohols and their key properties?
-compounds containing at least one hydroxy group (oh)
-polar with electronegative oxygen
-able to form hydrogen bonds
-water soluble if small hydrocarbon framework
-higher melting/boiling points than alkanes
-pka around 16-20 (significantly lower)
what are the three types of alcohols?
primary alcohol, secondary alcohol, and tertiary alcohol
what can primary alcohols be oxidized to?
aldehydes and carboxylic acids
what can secondary alcohols be oxidized to?
ketones
can tertiary alcohols be oxidized?
no, tertiary alcohols cannot be oxidized
what are ethers?
compounds containing an ether group where an oxygen atom is connected to two carbon atoms via single bonds (r-o-r')
what are the key properties of ethers?
-polar group but less than alcohols
-able to act as hydrogen donor but not acceptor
-melting and boiling points between alkanes and alcohols
-sp³ hybridized o atom
-chemically stable c-o bonds
how are ethers formed?
dehydration of two alcohols yields ethers
ether formation diagram
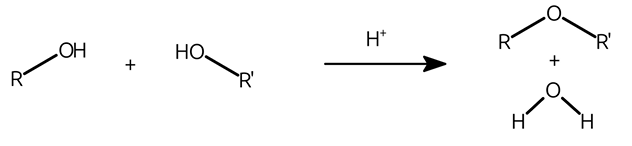
what are examples of biological ethers?
sucrose (glucose + fructose) and lactose (galactose + glucose)
what are thiols and thioethers?
the sulfur equivalents of alcohols and ethers where oxygen is replaced by sulfur
how do thiols differ from alcohols in terms of atomic properties?
sulfur has larger covalent radius and is less electronegative (2.58 vs 3.44) due to lower effective charge
compare the bond lengths between methanol and methanethiol
o-h bond is 0.96 å in methanol, while s-h bond is 1.33 å in methanethiol (longer)
compare the pka of methanol and methanethiol
-methanol has pka of 15.5, while methanethiol has pka of 10.4
-thiols can be deprotonated much more readily
how do thiols compare to alcohols in boiling points?
-thiols have lower boiling points than alcohols as interactions are weaker
-still much higher than alkanes
what are the biological examples of thiols and thioethers?
two amino acids - cysteine (thiol) and methionine (thioether)
what is a practical application of thiols in research?
3-maleimido proxyl (5-msl) can be used as a spin label for spectroscopy, enabling folding studies
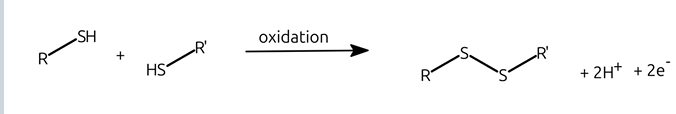
how do disulfide bridges form?
formed in oxidizing conditions by two thiols. in proteins, formed by cysteines in close proximity
disulphide formation diagram
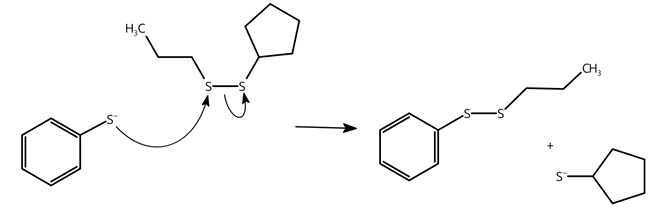
can disulfide bonds exchange?
yes, disulfide bonds can exchange with deprotonated thiols
disulfide bonds exchange diagram
what is the preferred dihedral angle for c-s-s-c bonds?
configurations around 90°. flattening towards 0° or 180° increases reactivity
how do s-s bonds compare to c-c bonds?
s-s bonds are around 0.5 å longer than c-c and about 40% weaker in bond dissociation energy
what is disulfide-thiol exchange an example of?
a special case of the general possibility of bond breakage through nucleophilic attack
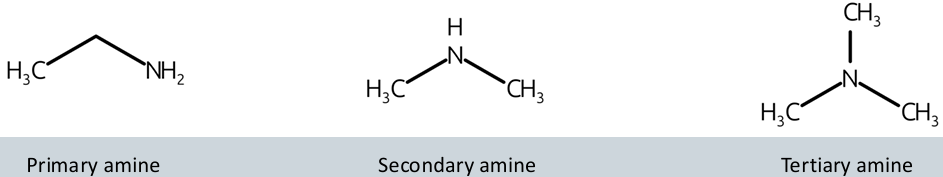
what are amines?
-the nitrogen equivalents of alcohols and ethers
-group v element (not group vi like oxygen),
-can have three single bonds in its neutral state
what are the three types of amines?
primary amine, secondary amine, and tertiary amine
how do amines compare to alcohols in hydrogen bonding?
-amines can form hydrogen bonds
-these are weaker than for oh due to the difference in electronegativity
compare hydrogen bonding ability across primary, secondary, and tertiary amines
primary amines form more hydrogen bonds than secondary amines, which in turn have more hydrogen bonds than tertiary amines
how does water solubility change from primary to tertiary amines?
solubility in water decreases from primary to tertiary amines
what are typical pka values for protonated amines?
pka values for r-nh₃⁺ is around 10 for r=me and 4.6 for r=phenyl
what is the common charge state of amines in biological systems?
amines are often positively charged by being protonated
what are aldehydes and ketones?
compounds containing carbonyl groups (c=o) embedded
-between two carbon atoms (ketone)
-between a carbon and a hydrogen atom (aldehyde)
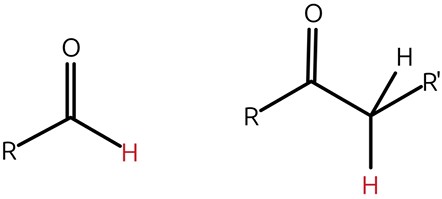
how are ketones and aldehydes formed?
ketones are formed by oxidation of secondary alcohols, while aldehydes are oxidized primary alcohols
what are the key features of the carbonyl group?
-carbon atom double bonded to oxygen
-both atoms are sp² hybridized
-bond is polarized with c being partially positive
-bond angles around 120°
-pka of aldehyde h is 17 and h in ketones is 20
are aldehydes or ketones more reactive?
aldehydes are more reactive than ketones
are aldehydes and ketones soluble in water?
yes, both are soluble in water as they can form hydrogen bonds with solvent
what is keto-enol tautomerism?
an equilibrium between the keto form (carbonyl) and enol form (alcohol with double bond) of carbonyl compounds
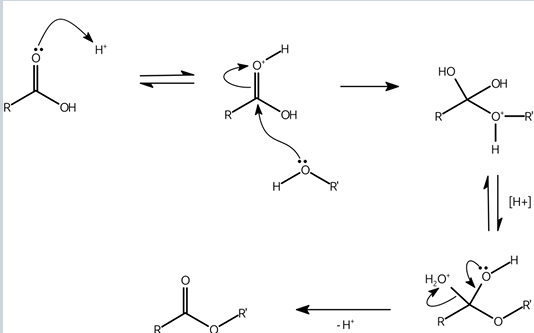
what are hemiacetals and hemiketals?
intermediate compounds formed when aldehydes or ketones react with alcohols (one equivalent)
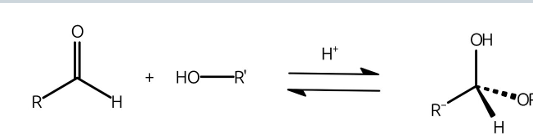
what are carboxylic acids?
compounds derived from aldehydes by oxidation with a hydroxyl group on the carbonyl carbon
what are the key properties of carboxylic acids?
-deprotonation of hydroxyl group occurs readily leading to negative charge
-can form hydrogen bonds in protonated form
-soluble in water in deprotonated phase
-key group to form other functional groups (e.g., esters and amides)
what are typical pka values for carboxylic acids?
acetic acid (pka 4.76), formic acid (pka 3.90), trifluoroacetic acid (pka 1.99), chloroacetic acid (pka 2.10), propionic acid (pka 4.07)
what amino acids are based on carboxylic acids?
aspartic acid and glutamic acid are both based on carboxylic acids and are deprotonated at physiological ph
how are esters formed?
-by condensation of an alcohol and a carboxylic acid
-using dehydration agents and acid or base catalysis
ester formation diagram
what is commonly used instead of carboxylic acid when synthesizing esters?
acyl chlorides (r-(c=o)-cl) are often used instead of the acid
how are amides formed?
by condensation of a carboxylic acid and an amine
can amides form hydrogen bonds?
yes, amides can form hydrogen bonds, but in some cases only as acceptors
under what conditions does amide hydrolysis occur?
hydrolysis (breaking the amide bond between c=o and n) generally only happens in very acidic or basic conditions
amide diagram structure
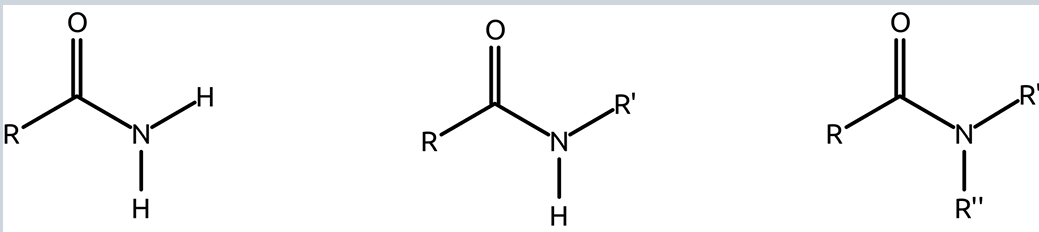
2 examples of common groups in biochemistry
-guanidine
-imidazole
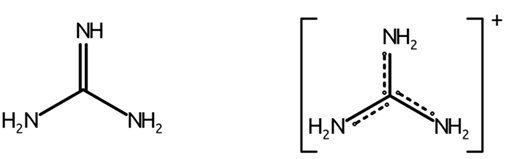
what is guanidine
-strong base
-commonly found as guanidium cation
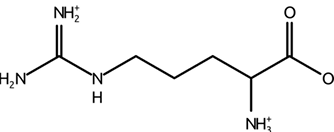
-found as a functional group in arginine
what is the pKa of guanidium cation
13.6
guanidine & guanidium cation structure
arginine structure
what kind of agent is guanidine
-guanidinium chloride (GdnHCl) denatures proteins with an approx linear relationship between conc and free energy to denature a protein
-at 6M, most protein in aqueous solutions are denatured by it
what is imidazole
-aromatic compound with 2 tautomeric forms
-where H can be bound to either N
2 structures of imidazole
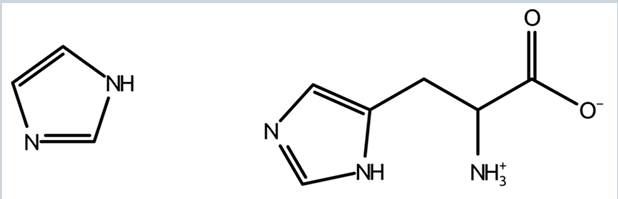
what does imidazole do
-displays amphoteric behaviour; acts as both base and acid
-N w/out H can be protonated
-N w/ H can be deprotonated
what is the biological significance of imidazole?
this ph dependency is observed in histidine and used in biological systems to move protons around active sites
how does the length of lipid tails affect melting temperature?
-longer tails require more energy for melting
-mp ranges from -1°c to 55°c (comparing shorter vs longer tails)
how do double bonds in lipid tails affect melting temperature?
-double bonds increase flexibility of the tail
-mp of -20°c vs 55°c without double bonds (double bonds lower melting temperature)
how do different head groups affect lipid melting?
changes in interactions of head groups affect melting
what are the four main types of organic reactions?
-addition (combination of multiple molecules)
-elimination (splitting of a molecule into multiple)
-substitution (replacing substituents)
-rearrangements
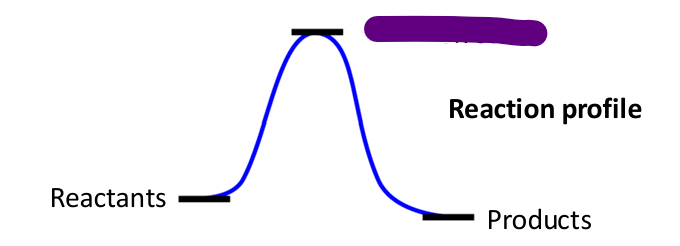
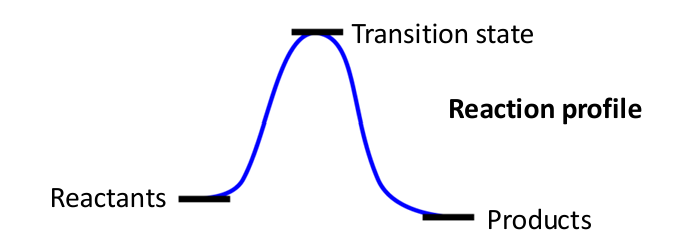
what determines the reaction outcome thermodynamically?
the relative free energy of reactants and products determines the reaction outcome
what determines the time scale for chemical transformation?
the energy barrier separating reactants and products determines the time scale (reaction kinetics)
what is a reaction mechanism?
the step-by-step pathway of how a reaction proceeds, which may involve intermediates and multiple steps
why do chemical reactions have activation barriers?
-when bonds are broken (requires energy) or when molecules approach each other, electron clouds repel
-overcoming this repulsion requires activation energy
how does charge attraction affect activation energy?
-charged and polarized groups exhibit electrostatic attraction pulling groups together
-this lowers activation energies and helping bring molecules together
what drives specific reactions and determines their mechanisms?
the interaction between orbitals drives specific reactions, determines their mechanisms, and leads to specific products
what are chemical reactions fundamentally?
-chemical reactions happen when electrons move between atoms/molecules to break or form chemical bonds
what is a nucleophile?
-the species donating electrons
-any molecule or ion with a free pair of electrons or at least one unsaturated bond can act as nucleophile
-nucleophiles are lewis bases (electron donors)
what is an electrophile?
-the acceptor of electrons
-species attracted to negatively charged or electron-rich atoms
-they are lewis acids (electron acceptors)
what are typical examples of electrophiles?
-cations such as h⁺
-molecules with empty orbitals
-molecules with low lying anti-bonding orbitals
what are typical examples of nucleophiles?
ions such as:
-hydroxide
-cyanide
-bromide
-groups with lone pairs like alcohols or thiols
-double bonds
why can carbonyl groups act as electrophiles?
-the bond between c and o is polarized due to poor energy match between interacting orbitals
-creating a partial positive charge on the carbon
what happens when a nucleophile attacks a carbonyl group?
-as the lumo (lowest unoccupied molecular orbital) is the π*, the addition of a nucleophile will break the double bond
what determines if the reaction proceeds with elimination to reform the carbonyl?
-depends on the nature of x (the leaving group)
-generally requires x to be stable on its own
what makes a carbonyl group more reactive to nucleophilic attack?
-more positive the partial charge on the c atom in the carbonyl group, the more likely the reaction becomes. similarly, the more stable the leaving group is, the better (judged by pka)
rank carbonyl compounds by reactivity to nucleophilic attack
most reactive: acyl chloride > anhydride > aldehyde ≥ ketone > ester > amide > carboxylate: least reactive
when can sp³ carbons act as electrophiles?
if the single bond between a sp³ carbon and a heteroatom is polarized enough, the carbon becomes partially positively charged
what happens when a nucleophile attacks an sp³ carbon electrophile?
the nucleophile attacks the carbon and forms a bond while kicking out the polar heteroatom (leaving group)
why does nucleophilic attack on sp³ carbon lead to substitution?
as the lumo is the σ*, the addition of a nucleophile will always break the existing single bond, leading to a substitution reaction
what factors determine the mechanism of nucleophilic substitution?
the leaving group, the geometry of the sp³ carbon, and the nucleophile. the mechanism will impact the stereochemistry
what factors determine electrophile strength?
electronegativity, geometry, and bonding. high electronegativity polarizes bonds with no delocalization making stronger electrophiles. delocalization of lone pairs on n or o leads to less polar carbon
what determines nucleophile strength?
the nature of the lone pair or charge, orbital size (large orbitals react with sp³ c, small with c=o), whether the nucleophile is charged and/or basic. broadly, the higher the pka of ah, the stronger a nucleophile is a⁻
what makes a good leaving group?
determined by its stability once it leaves the molecule. the lower the pka of ah, the better a leaving group a⁻ can be
compare leaving group ability of different groups
cl⁻ is good (hcl pka -7), pho⁻ is better than ro⁻ (pka 10 vs 15), r⁻ and nh₂⁻ are very poor (pka over 40 and over 20), so ketones and amides generally don't react this way
how do functional groups enable specific behavior?
functional groups enable specific, characteristic physical and chemical behavior through distinct interactions (dipole interactions, hydrogen bonds) and distinct geometries/bonds that enable selective reactivity
how can reactions be understood fundamentally?
reactions can be seen as the interaction of electron-rich compounds (nucleophiles) with electron-poor compounds (electrophiles), explained by considering properties of atoms and bonds in reactants
what determines the physical properties of compounds with different functional groups?
different functional groups enable different interactions such as dipole interactions or hydrogen bonds, which then determine the physical properties
why do amino acid side chains have different properties?
the amino acid side chains have distinct functional groups that lead to specific properties